4. Determine if a chemical reaction occurs in the following. If a reaction occurs identify the solid. a. Aqueous cobalt (III) bromide and aqueous potassium sulfide are mixed 2CóBrs (an) + 3 KzS (aar) Chemical reaction CO2$a Cooạit(N) SUIfide b. Äqueous beryllium iodide is mixed with aqueous silver sulfate BEİ2 laq) + Ag2 SO4 Caq) Chumica reaction is DesQu Beylliym ucate AaI siver ledide c. Aqueous lead (II) chlorate is mixed with aqueous ammonium sulfate Po (ocis)2l6)+ (NHa)z SOn Ca)-> Po (SOu)s+2 NH4CIO scan) -> CO2 S3ls) + 6 KBr (aa) -> BeSouls)+ 2 Ag I) chemical reachioa s Palso lead.sugate
4. Determine if a chemical reaction occurs in the following. If a reaction occurs identify the solid. a. Aqueous cobalt (III) bromide and aqueous potassium sulfide are mixed 2CóBrs (an) + 3 KzS (aar) Chemical reaction CO2$a Cooạit(N) SUIfide b. Äqueous beryllium iodide is mixed with aqueous silver sulfate BEİ2 laq) + Ag2 SO4 Caq) Chumica reaction is DesQu Beylliym ucate AaI siver ledide c. Aqueous lead (II) chlorate is mixed with aqueous ammonium sulfate Po (ocis)2l6)+ (NHa)z SOn Ca)-> Po (SOu)s+2 NH4CIO scan) -> CO2 S3ls) + 6 KBr (aa) -> BeSouls)+ 2 Ag I) chemical reachioa s Palso lead.sugate
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter15: Equilibria Of Other Reaction Classes
Section: Chapter Questions
Problem 51E: Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production...
Related questions
Question
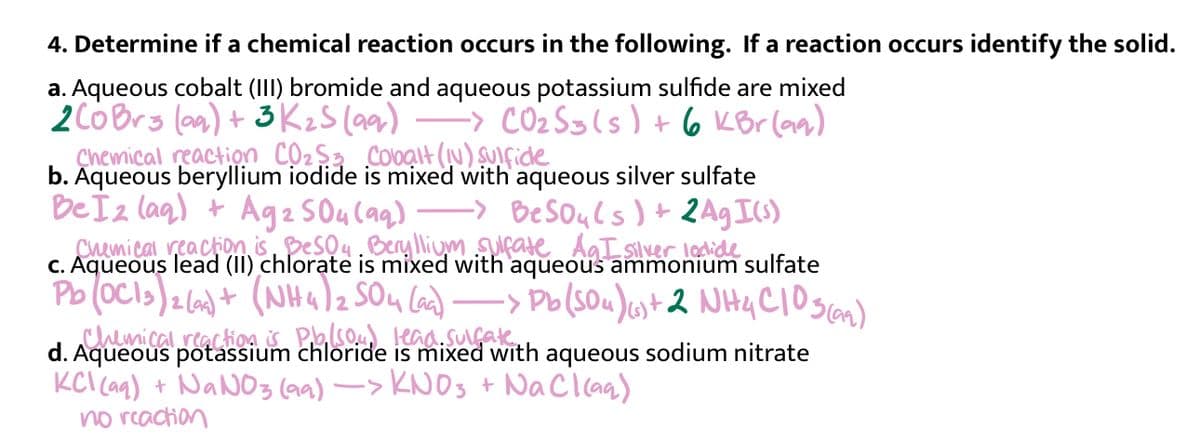
Transcribed Image Text:4. Determine if a chemical reaction occurs in the following. If a reaction occurs identify the solid.
a. Aqueous cobalt (III) bromide and aqueous potassium sulfide are mixed
-> CO2 Sgls) + 6 KBr (aa)
2CóBrs (a) + 3 KzS (aar)
Çhemical reaction Cộ2$3 Colbạlt (N) SUIfide
b. Aqueous beryllium iodide is mixed with aqueous silver sulfate
BeSouls )+ 2Ag I)
Chemical reaction isDesou. Beryllium surate AgT silver lodide
Be Ïz lag) + Ag2 SO4 Caq)
C. Aqueous lead (II) chlorate is
Pb (oCls)2(6)+ (NHa)z SOu Cad)-
chemical.reactio4 S Pso kaa.suake
d. Aqueous potassium chloride is
KCl Ca) + NANO3 (aa) -> KNOS + NaCl caa)
no reaction
with aqueous ammonium sulfate
-> Po(SOu)ust2 NH4CIoscan)
with aqueous sodium nitrate

Transcribed Image Text:5. For the reactions in problem 4 write a balanced molecular equation, balance ionic equation, net ionic
equation and identify the spectator ions. Include physical states.
6. A recipe for Reuben sandwiches calls for 2 tablespoons butter, 8 slices rye bread, 16 slices deli sliced corned beef, 24
slices Swiss cheese, 1 cup sauerkraut, and 1/2 cup Thousand Island dressing. How many sandwiches can be made from
8 tablespoons butter, 15 slices rye bread, 24 slices deli sliced corned beef, 40 slices Swiss cheese, 1 1/2 cup sauerkraut,
and 3 cup Thousand Island dressing.
a. What is the limiting reactant?
b. How much is left over of the other ingredients?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning