4. Determine the initial concentrations of Fe" and SCN (before any reaction) and the equilibrium concentrations of FESCN (after the reaction has reached equilibrium) for the rest of the tubes in Part A. Refer to the volumes of Fe(NO). KSCN and water listed on page 3 (steps 2 and 3). Note that you already did tube 5 and that ALL tubes have the same [Fe"Jnit. [Fe linit [FESCN"leg Tube [SCN Jinit 2. 3 4. 6. 7.
4. Determine the initial concentrations of Fe" and SCN (before any reaction) and the equilibrium concentrations of FESCN (after the reaction has reached equilibrium) for the rest of the tubes in Part A. Refer to the volumes of Fe(NO). KSCN and water listed on page 3 (steps 2 and 3). Note that you already did tube 5 and that ALL tubes have the same [Fe"Jnit. [Fe linit [FESCN"leg Tube [SCN Jinit 2. 3 4. 6. 7.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.37PAE: Again the experiment in Exercise 12.33 was redesigned. This time, 0.15 mol each of N, and O2 was...
Related questions
Question
5
Please answer #4 and #5
If any info is missing that is needed, leave a comment and I will update that.
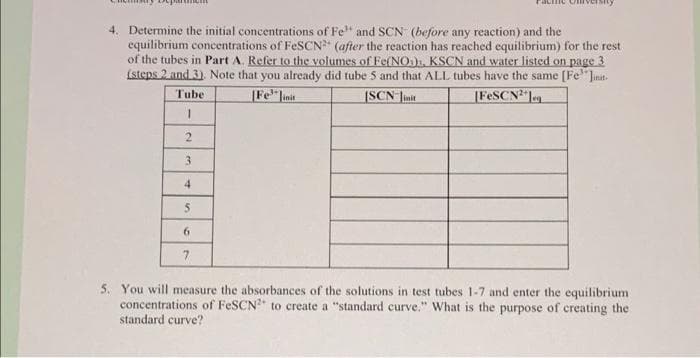
Transcribed Image Text:4. Determine the initial concentrations of Fe and SCN (before any reaction) and the
equilibrium concentrations of FeSCN" (after the reaction has reached equilibrium) for the rest
of the tubes in Part A. Refer to the volumes of Fe(NO), KSCN and water listed on page 3
Lsteps 2 and 3). Note that you already did tube 5 and that ALL tubes have the same [Fe'"Jinit.
ISCN Jinit
|Fe"linit
|FESCN leg
Tube
3
4
6.
7
5. You will measure the absorbances of the solutions in test tubes 1-7 and enter the equilibrium
concentrations of FeSCN to create a "standard curve." What is the purpose of creating the
standard curve?
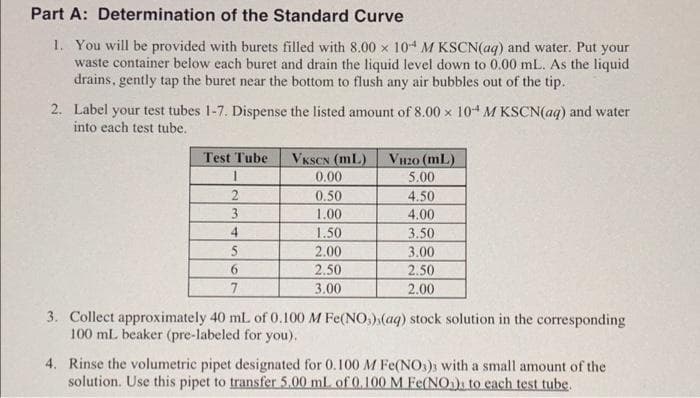
Transcribed Image Text:Part A: Determination of the Standard Curve
1. You will be provided with burets filled with 8.00 x 104 M KSCN(aq) and water. Put your
waste container below each buret and drain the liquid level down to 0.00 mL. As the liquid
drains, gently tap the buret near the bottom to flush any air bubbles out of the tip.
2. Label your test tubes 1-7. Dispense the listed amount of 8.00 x 104 M KSCN(aq) and water
into each test tube.
Test Tube
VKSCN (mL)
VH2O (mL)
0.00
5.00
0.50
1.00
2
4.50
3
4.00
4.
1.50
2.00
3.50
5
3.00
2.50
3.00
6.
2.50
7
2.00
3. Collect approximately 40 ml of 0.100 M Fe(NO,).(aq) stock solution in the corresponding
100 mL beaker (pre-labeled for you).
4. Rinse the volumetric pipet designated for 0.100 M Fe(NOs); with a small amount of the
solution. Use this pipet to transfer 5.00 ml. of 0.100 M Fe(NO)s to each test tube.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning