4.) For the following equilibrium reaction state what happens when the following reagents are added N2OH(ag) + HC(ag) HOH(ag+ NaCl(ag) +4 a.) Mg(OH)2 b.) KCI c.) KBr d.) Heat (A) e.) CH:CH.COH (an acid) £.) decrease in temp.
4.) For the following equilibrium reaction state what happens when the following reagents are added N2OH(ag) + HC(ag) HOH(ag+ NaCl(ag) +4 a.) Mg(OH)2 b.) KCI c.) KBr d.) Heat (A) e.) CH:CH.COH (an acid) £.) decrease in temp.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter4: Polar Bonds, Polar Reactions
Section: Chapter Questions
Problem 46CTQ
Related questions
Question
Hi, I know this question has multiple parsers but it counts as one question. Thank you and I hope you have a wonderful day.

Transcribed Image Text:For the following questions state whether there would be a shift to the
right, left, more of a shift to the right or left, or nothing. If asked why a
reaction shifts in a particular reason the answer should be kept simple
because it decreases, increases, or does nothing to a specific reactant,
product, or nothing to the main reaction. State what the exact reactant.
For instance, the below reaction if something is added that decreases
NH,, then write “the reaction shifts to the left because it decreases
NH,".
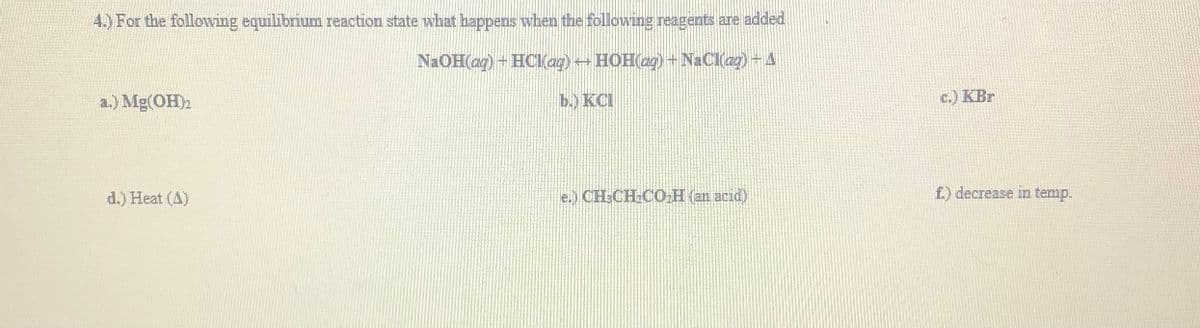
Transcribed Image Text:4.) For the followving equilibrium reaction state what happens when the following reagents are added
NAOH(ag) + HCI(ag) HOH(ag)+ NaClag)-4
a.) Mg(OH)2
b.) KCI
c.) KBr
d.) Heat (A)
e.) CH:CH.CO,H (an acid)
f.) decrease in temp.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning