Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 86GQ: In 1774, C. Scheele obtained a gas by reacting pyrolusite (MnO2) with sulfuric acid. The gas, which...
Related questions
Question
100%
Explain 4
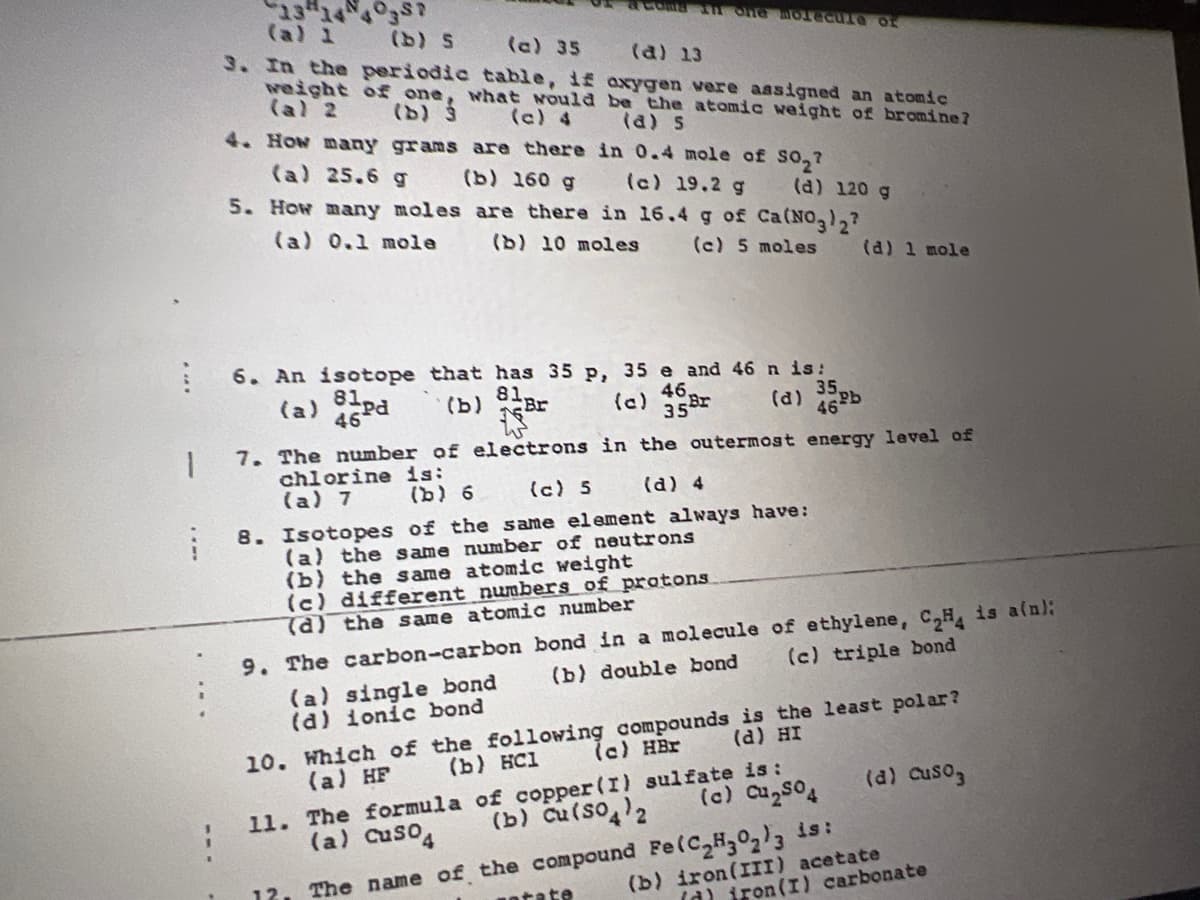
Transcribed Image Text:13 14 4 3s7
(a) 1
(b) S
(a) 35 (a) 13
3. In the periodic table, if axygen vere aasigned an atomic
weight of one, what would be the atomic weight of bromine?
(al 2
(b) 3
(c) 4
4. HOw many grams are there in 0.4 mole of so,?
(a) s
(a) 25.6 g
(b) 160 g
5. How many moles are there in 16.4 g of Ca(NO,),?
(c) 19.2 g
(d) 120 g
(a) 0.1 mole
(b) 10 moles
(c) 5 moles
(d) 1 mole
6. An isotope that has 35 p, 35 e and 46 n is:
81
(a) a5Pd
81
46
(b) Br
46,
(a) 16b
35.
7. The number of electrons in the outermost energy level of
chlorine is:
(a) 7
(b) 6
(c) 5
(d) 4
8. Isotopes of the same element always have:
(a) the same number of neutrons
(b) the same atomic weight
(c) different numbers of protons.
(a) the same atomic number
9. The carbon-carbon bond in a molecule of ethylene, C,H is aln:
(b) double bond
(c) triple bond
(a) single bond
(d) ionic bond
10. Which of the following compounds is the least polar?
(c) HBr
(d) HI
(b) HC1
11. The formula of copper (I) sulfate is:
(b) Cu(so2
(a) HF
(a) cuso3
(c) Cu2s04
(a) Cuso4
The name of the compound Fe(C H,0,)3 is:
(b) iron(III) acetate
12.
ntate
(d) iron(I) carbonate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning