Determining the absorbance of known concentrations for Solution A: What is the most sensitive wavelength for Solution A (meaning highest absorbance) Using this wavelength, we now place known concentrations of Sample A in the Spec 20 ma- chine and see the absorbances. Sample A concentrations 0.000Molar (distilled water) Absorbance using Spec 20 Instrument 0.000 0.020 Molar 0.050 Molar 0.100 Molar 0.150 0.382 0.550 0.150 Molar 0.950 0.200 Molar 1.230 What is the independent & dependent variables? What variables go on what axis? Graph the sample concentrations vs the absorbances on the graph paper be- Does this a direct or inverse relationship? low: Is this graph more linear or nonlinear? Determining the concentration of an unknown sample using the standard curve from above. Last initial Unknown Concentration from the graph Absorbance 563 799 199 A6 0.800 219 329 317 N2 0.720 491 968 791 LS 0.650 823 790 193 094 585 181 Y-4 1.150 T-8 0.850 373 556 036 M-12 0.960
Determining the absorbance of known concentrations for Solution A: What is the most sensitive wavelength for Solution A (meaning highest absorbance) Using this wavelength, we now place known concentrations of Sample A in the Spec 20 ma- chine and see the absorbances. Sample A concentrations 0.000Molar (distilled water) Absorbance using Spec 20 Instrument 0.000 0.020 Molar 0.050 Molar 0.100 Molar 0.150 0.382 0.550 0.150 Molar 0.950 0.200 Molar 1.230 What is the independent & dependent variables? What variables go on what axis? Graph the sample concentrations vs the absorbances on the graph paper be- Does this a direct or inverse relationship? low: Is this graph more linear or nonlinear? Determining the concentration of an unknown sample using the standard curve from above. Last initial Unknown Concentration from the graph Absorbance 563 799 199 A6 0.800 219 329 317 N2 0.720 491 968 791 LS 0.650 823 790 193 094 585 181 Y-4 1.150 T-8 0.850 373 556 036 M-12 0.960
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.10QAP
Related questions
Question
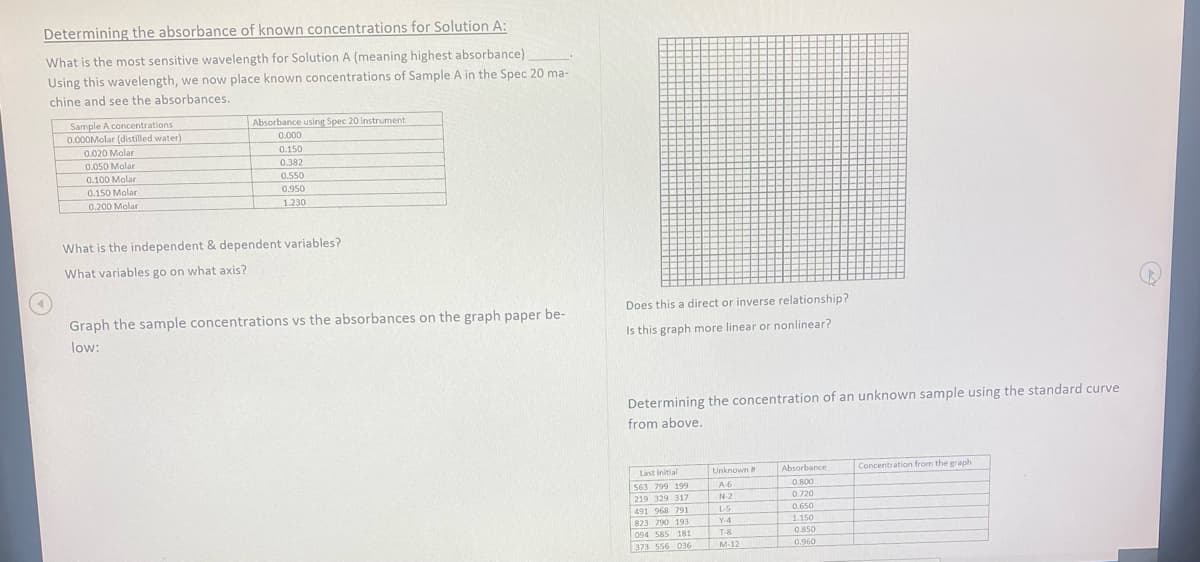
Transcribed Image Text:Determining the absorbance of known concentrations for Solution A:
What is the most sensitive wavelength for Solution A (meaning highest absorbance)
Using this wavelength, we now place known concentrations of Sample A in the Spec 20 ma-
chine and see the absorbances.
Sample A concentrations
0.000Molar (distilled water)
0.020 Molar
0.050 Molar
Absorbance using Spec 20 instrument
0.000
0.150
0.382
0.100 Molar
0.550
0.150 Molar
0.950
0.200 Molar
1.230
What is the independent & dependent variables?
What variables go on what axis?
Graph the sample concentrations vs the absorbances on the graph paper be-
Does this a direct or inverse relationship?
low:
Is this graph more linear or nonlinear?
Determining the concentration of an unknown sample using the standard curve
from above.
Last initial
Unknown
Absorbance
Concentration from the graph
563 799 199
A6
0.800
219 329 317
N-2
0.720
491 968 791
L-S
0.650
823 790 193
Y-4
1.150
094 585 181
T-8
0.850
373 556 036
M-12
0.960
Transcribed Image Text:Application question using the Spec 20 machine:
You want to determine if the toxic metal Lead (Pb) was
your drinking water and if the con-
centration was a high enough Molarity to cause health problems. Using the Spec 20 instru-
ment, how would you set up a procedure to identify the metal and determine the concentra-
tion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning