4. In Figure 4 below, a graph shows the relationship between mass and volume for two substances, A and B. Use the graph to answer questions about these two substances. FIGURE 1: Mass and Volume Reļationships a) Which substance is more dense, substance A or substance B? Explain your reasoning. Substahde A 60- b) Find the slope of the line for both A and B using correct units. State the physical meaning of the slope ("For every...") for each substance. slope A 10 For crery... 30 SubstanceB X2-X 10 Slope B volumel(mL For every... c) Water has a density of 1.00 g/mL. Draw the line representing water on the graph in Figure 4. d) Determine whether substance A and B will sink or float when placed in a bucket of water. Substance A: sink float Substance B: sink float (circle correct response)
4. In Figure 4 below, a graph shows the relationship between mass and volume for two substances, A and B. Use the graph to answer questions about these two substances. FIGURE 1: Mass and Volume Reļationships a) Which substance is more dense, substance A or substance B? Explain your reasoning. Substahde A 60- b) Find the slope of the line for both A and B using correct units. State the physical meaning of the slope ("For every...") for each substance. slope A 10 For crery... 30 SubstanceB X2-X 10 Slope B volumel(mL For every... c) Water has a density of 1.00 g/mL. Draw the line representing water on the graph in Figure 4. d) Determine whether substance A and B will sink or float when placed in a bucket of water. Substance A: sink float Substance B: sink float (circle correct response)
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 56QAP: Magnesium chloride is an important coagulant used in the preparation of tofu from soy milk. Its...
Related questions
Question
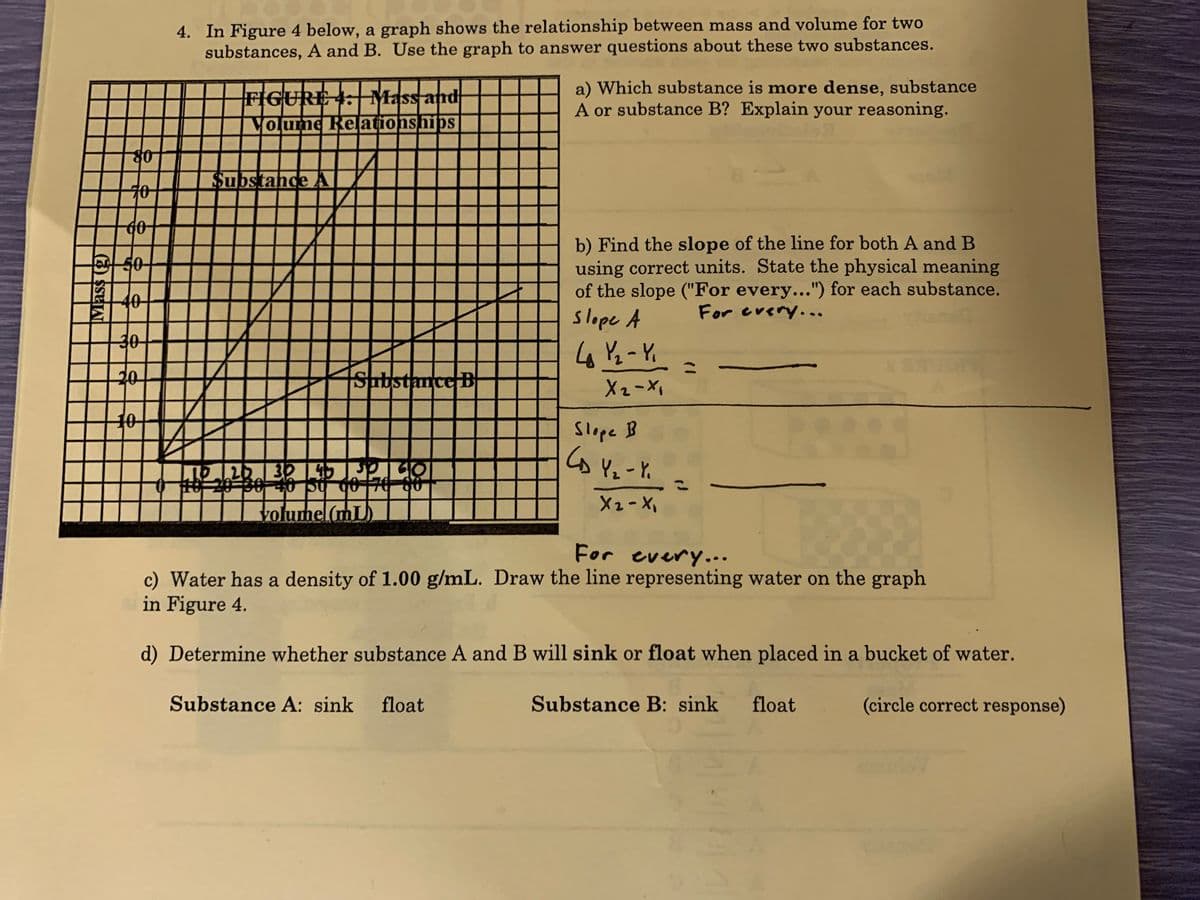
Transcribed Image Text:4. In Figure 4 below, a graph shows the relationship between mass and volume for two
substances, A and B. Use the graph to answer questions about these two substances.
FIGURE 4: Mass and
VOume Reationships
a) Which substance is more dense, substance
A or substance B? Explain your reasoning.
४०
Substande A Z
60
b) Find the slope of the line for both A and B
using correct units. State the physical meaning
of the slope ("For every...") for each substance.
slope A
50-
40
For cvery...
%3D
20
Substance B
Slope B
Y2-Y.
X2-X
volumel(mL)
For every...
c) Water has a density of 1.00 g/mL. Draw the line representing water on the graph
in Figure 4.
d) Determine whether substance A and B will sink or float when placed in a bucket of water.
Substance A: sink float
Substance B: sink
float
(circle correct response)
Mass (g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning