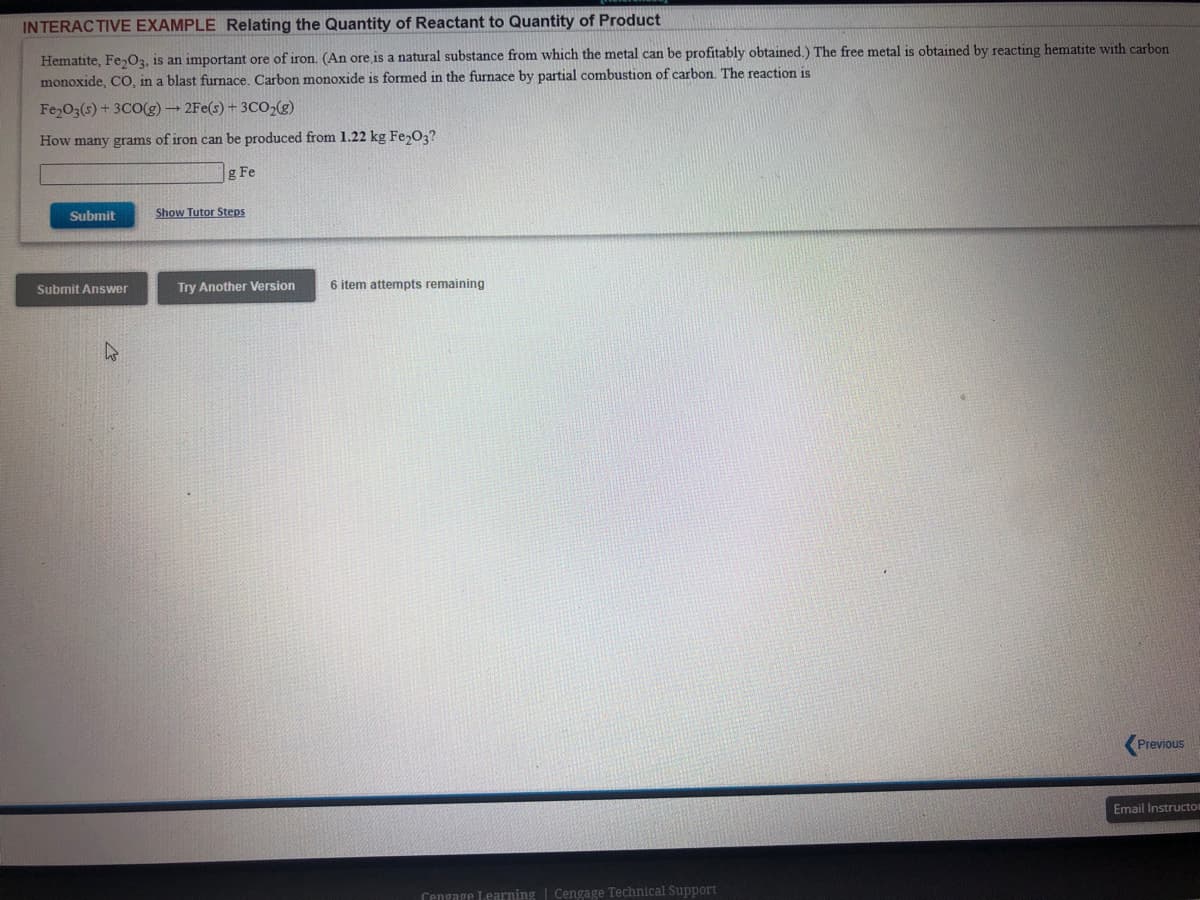
Hematite, Fe,O3, is an important ore of iron. (An ore is a natural substance from which the metal can be profitably obtained.) The free metal is obtained by reacting hematite with carbon monoxide, CO, in a blast furnace. Carbon monoxide is formed in the furnace by partial combustion of carbon. The reaction is Fe O3(s) + 3CO(g) 2Fe(s) + 3CO(g) How many grams of iron can be produced from 1.22 kg Fe,O3? Fe
Hematite, Fe,O3, is an important ore of iron. (An ore is a natural substance from which the metal can be profitably obtained.) The free metal is obtained by reacting hematite with carbon monoxide, CO, in a blast furnace. Carbon monoxide is formed in the furnace by partial combustion of carbon. The reaction is Fe O3(s) + 3CO(g) 2Fe(s) + 3CO(g) How many grams of iron can be produced from 1.22 kg Fe,O3? Fe
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.105PAE
Related questions
Question
Transcribed Image Text:IN TERACTIVE EXAMPLE Relating the Quantity of Reactant to Quantity of Product
Hematite, Fe,O3, is an important ore of iron. (An ore is a natural substance from which the metal can be profitably obtained.) The free metal is obtained by reacting hematite with carbon
monoxide, CO, in a blast furnace. Carbon monoxide is formed in the furnace by partial combustion of carbon. The reaction is
Fe O3(s) + 3CO(g) 2Fe(s) + 3CO,(g)
How many grams of iron can be produced from 1.22 kg Fe,03?
g Fe
Submit
Show Tutor Steps
Submit Answer
Try Another Version
6 item attempts remaining
Previous
Email Instructom
Cengage Learning | Cengage Technical Support
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning