4. Oxidation: Zn(s) → Zn?+ (aq) + 2e E°ox = + 0.76V Reduction: Cu² (aq) + 2e → Cu(s) E'red = + 0.34 Net reaction: Zn(s) + Cu² (aq) → Cu(s) +Zn?* (aq) a) What is the E°an? b) Calculate the AG° and determine whether the reaction is spontaneous or not spontaneous.
4. Oxidation: Zn(s) → Zn?+ (aq) + 2e E°ox = + 0.76V Reduction: Cu² (aq) + 2e → Cu(s) E'red = + 0.34 Net reaction: Zn(s) + Cu² (aq) → Cu(s) +Zn?* (aq) a) What is the E°an? b) Calculate the AG° and determine whether the reaction is spontaneous or not spontaneous.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 71E: The overall reaction in the lead storage battery is Pb(s)+PbO2(s)+2H+(aq)+2HSO4(aq)2PbSO4(s)+2H2O(l)...
Related questions
Question
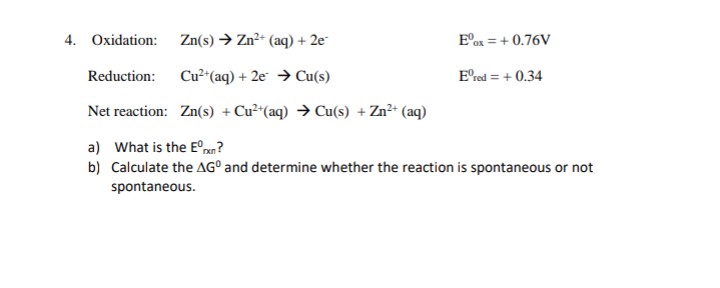
Transcribed Image Text:4. Oxidation:
Zn(s) → Zn2+ (aq) + 2e
E°ox = + 0.76V
Reduction:
Cu²*(aq) + 2e → Cu(s)
E'red = + 0.34
Net reaction: Zn(s) + Cu²*(aq) → Cu(s) + Zn²* (aq)
a) What is the E°xn?
b) Calculate the AG° and determine whether the reaction is spontaneous or not
spontaneous.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning