4. The total number of the accessible microscopic states of the Boltzmann gas, with energy E and number of particles N, may be written as N! N(E,N) = E N!N2! - .. with the restrictions EN; = N and DGN; = E. Except for an additive constant, show that the entropy per par- ticle is given by
4. The total number of the accessible microscopic states of the Boltzmann gas, with energy E and number of particles N, may be written as N! N(E,N) = E N!N2! - .. with the restrictions EN; = N and DGN; = E. Except for an additive constant, show that the entropy per par- ticle is given by
Algebra & Trigonometry with Analytic Geometry
13th Edition
ISBN:9781133382119
Author:Swokowski
Publisher:Swokowski
Chapter5: Inverse, Exponential, And Logarithmic Functions
Section5.6: Exponential And Logarithmic Equations
Problem 64E
Related questions
Question
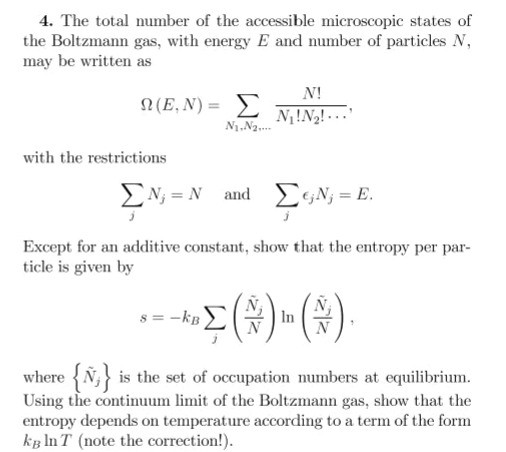
Transcribed Image Text:4. The total number of the accessible microscopic states of
the Boltzmann gas, with energy E and number of particles N,
may be written as
N!
Ω (Ε, N) = Σ
N!N2! ...
N1.N2.
with the restrictions
EN; = N and
EGN; = E.
Except for an additive constant, show that the entropy per par-
ticle is given by
s = -kB
In
where {Ñ;} is the set of occupation numbers at equilibrium.
Using the continuum limit of the Boltzmann gas, show that the
entropy depends on temperature according to a term of the form
kg In T (note the correction!).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage

Algebra & Trigonometry with Analytic Geometry
Algebra
ISBN:
9781133382119
Author:
Swokowski
Publisher:
Cengage