4. The work done in the isothermal, reversible expansion or compression of an ideal gas from volume V, to volume V, is given by the equation V2 w = -nRT In V1 where n is the number of moles of the gas, R is the gas constant = 8.314 J/mol · K, and T is the absolute temperature. Find the work done in the isothermal, reversible expansion of 1.00 mole of an ideal gas at 300K from a volume of 3.00 liters to a volume of 10.00 liters.
4. The work done in the isothermal, reversible expansion or compression of an ideal gas from volume V, to volume V, is given by the equation V2 w = -nRT In V1 where n is the number of moles of the gas, R is the gas constant = 8.314 J/mol · K, and T is the absolute temperature. Find the work done in the isothermal, reversible expansion of 1.00 mole of an ideal gas at 300K from a volume of 3.00 liters to a volume of 10.00 liters.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.133QP: Dry ice is solid carbon dioxide; it vaporizes at room temperature and normal pressures to the gas....
Related questions
Question
100%
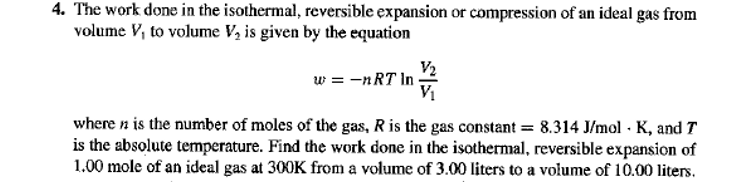
Transcribed Image Text:4. The work done in the isothermal, reversible expansion or compression of an ideal gas from
volume V, to volume V, is given by the equation
V2
w = -nRT In
V1
where n is the number of moles of the gas, R is the gas constant = 8.314 J/mol · K, and T
is the absolute temperature. Find the work done in the isothermal, reversible expansion of
1.00 mole of an ideal gas at 300K from a volume of 3.00 liters to a volume of 10.00 liters.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning