Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter7: Chemical Bonding And Molecular Structure
Section: Chapter Questions
Problem 7.99PAE
Related questions
Question
Please answer question 4
Transcribed Image Text:三
GD
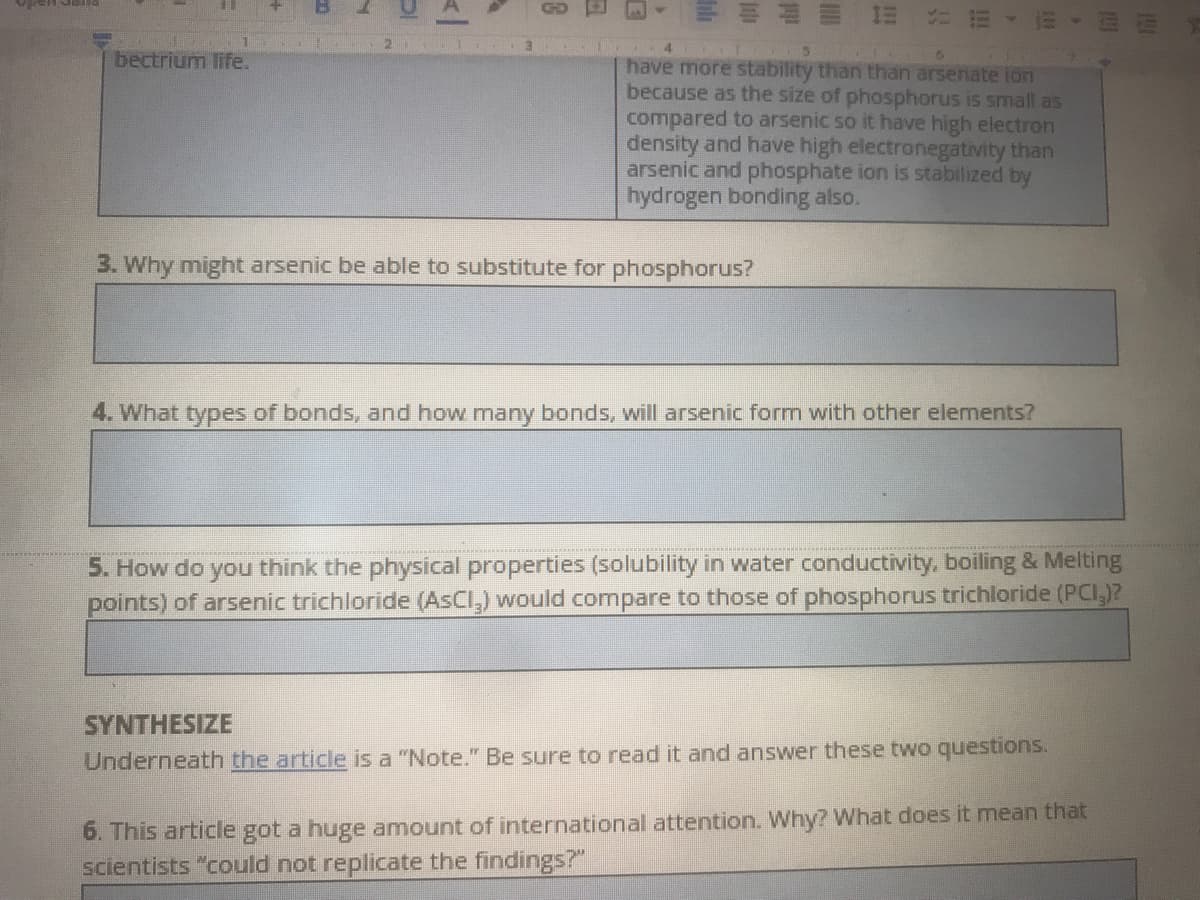
bectrium life.
have more stability than than arsenate ion
because as the size of phosphorus is small as
compared to arsenic so it have high electron
density and have high electronegativity than
arsenic and phosphate ion is stabilized by
hydrogen bonding also.
3. Why might arsenic be able to substitute for phosphorus?
4. What types of bonds, and how many bonds, will arsenic form with other elements?
5. How do you think the physical properties (solubility in water conductivity, boiling & Melting
points) of arsenic trichloride (AsCl,) would compare to those of phosphorus trichloride (PCI,)?
SYNTHESIZE
Underneath the article is a "Note." Be sure to read it and answer these two questions.
6. This article got a huge amount of international attention. Why? What does it mean that
scientists "could not replicate the findings?"
Transcribed Image Text:E Copof 11.4 Readin
W IM3 Desmos Activity
Copy of SAS 1-11- Go X
Copy of 11.4 Reading x
BazOlYc-iSooBC3Wzm7z2-CGittNfvx118hmpq4/edit
Ed Club
Math
it..
D (535) You Tube
arch for Life
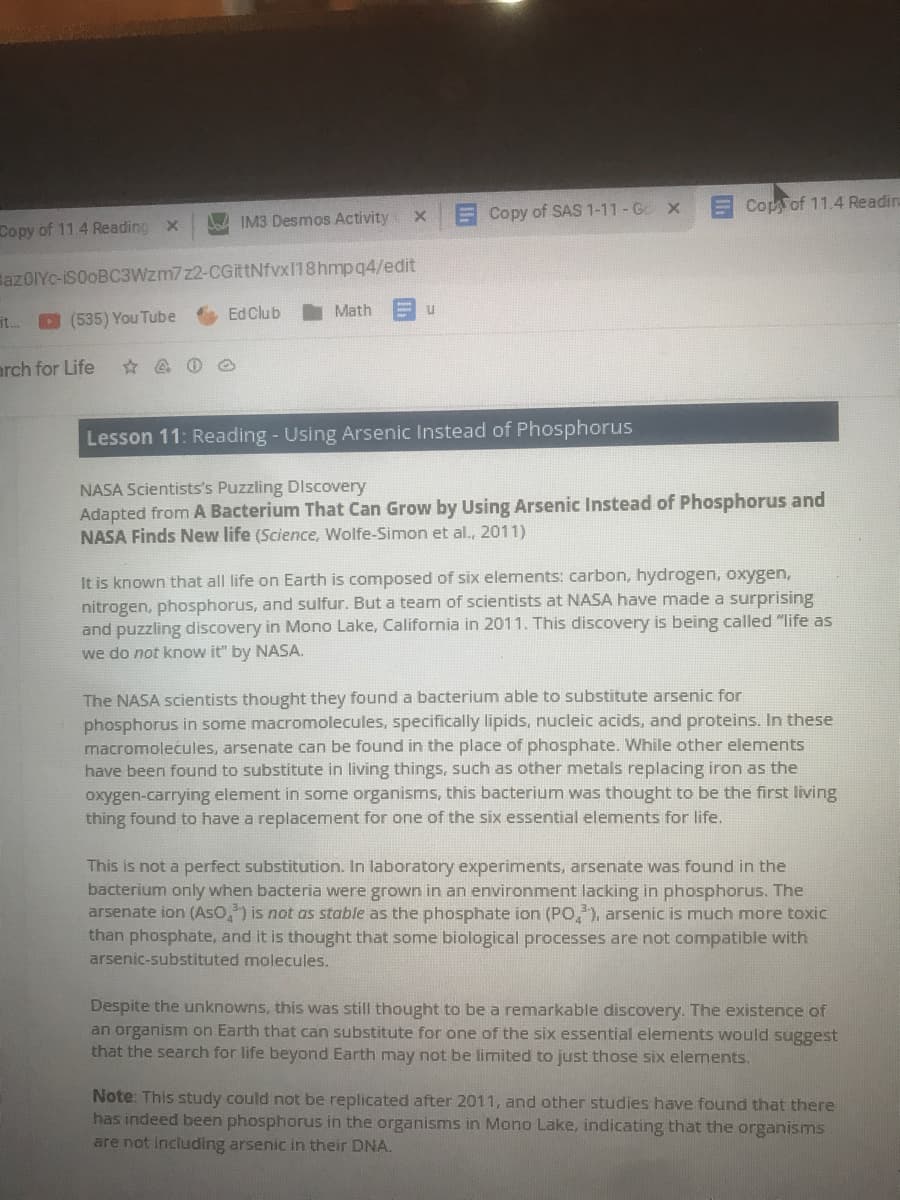
Lesson 11: Reading - Using Arsenic Instead of Phosphorus
NASA Scientists's Puzzling Discovery
Adapted from A Bacterium That Can Grow by Using Arsenic Instead of Phosphorus and
NASA Finds New life (Science, Wolfe-Simon et al., 2011)
It is known that all life on Earth is composed of six elements: carbon, hydrogen, oxygen,
nitrogen, phosphorus, and sulfur. But a team of scientists at NASA have made a surprising
and puzzling discovery in Mono Lake, California in 2011. This discovery is being called "life as
we do not know it" by NASA.
The NASA scientists thought they found a bacterium able to substitute arsenic for
phosphorus in some macromolecules, specifically lipids, nucleic acids, and proteins. In these
macromolećules, arsenate can be found in the place of phosphate. While other elements
have been found to substitute in living things, such as other metals replacing iron as the
oxygen-carrying element in some organisms, this bacterium was thought to be the first living
thing found to have a replacement for one of the six essential elements for life.
This is not a perfect substitution. In laboratory experiments, arsenate was found in the
bacterium only when bacteria were grown in an environment lacking in phosphorus. The
arsenate ion (AsO,) is not as stable as the phosphate ion (PO,), arsenic is much more toxic
than phosphate, and it is thought that some biological processes are not compatible with
arsenic-substituted molecules.
Despite the unknowns, this was still thought to be a remarkable discovery. The existence of
an organism on Earth that can substitute for one of the six essential elements would suggest
that the search for life beyond Earth may not be limited to just those six elements.
Note: This study could not be replicated after 2011, and other studies have found that there
has indeed been phosphorus in the organisms in Mono Lake, indicating that the organisms
are not including arsenic in their DNA.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning