Q: 2px зру 2.Draw an energy-level diagram for Polonium, Po (atomic number=84).
A:
Q: Write the balanced ionization reaction for HNO3
A: Ionisation reaction contain cation and anion when ionize.
Q: Buffer solutions Buffer solutions are produced by combining an acid with its conjugate base.…
A: Given: pH of solution = 9.0 pKa = 9.24 a). Total borate concentration = 10.0 mM c). sodium borate…
Q: What is the importance of the periodic table? What information can we gain from looking at the…
A: Importance of periodic table: Periodic table is defined as a table of chemical elements that are…
Q: We are currently working with diastereomeric products of acid-base reactions in my organic chemistry…
A: A Diastereomer is a stereoisomer with two or more stereo-centers, and the isomers are not mirror…
Q: Draw eugenol as a line structure. The double bonds of the benzene ring are fairly unreactive unless…
A: Eugenol is a phenolic compound,which is weakly acidic in nature.It is obtained from clove…
Q: 5 Give the IUPAC name for each of the following: a. H,C=CH–CH,—CH, b. CH3–C=C–CH,−CH,—CH–CH C. CH₂ 1…
A:
Q: Conventionally, we report reaction rates as absolute values (i.e., without reporting the sign).…
A: The given statement is True.
Q: 8. Assign oxidation numbers to each element in the following species: NH4Br A: -3, +1, -1
A: Given compound NH4Br
Q: Given: In a 10.0 mL 0.10 M CH3COOH solution, a 15 mL 1.00 M HCl solution was added. Compute for it's…
A: Given: Concentration of CH3COOH = 0.10 M Volume of CH3COOH = 10.0 mL Concentration of HCl = 1.0 M…
Q: How many moles of NO₂ would be required to produce 4.62 moles of HNO₃ in the presence of excess…
A:
Q: QUESTION 1 Predict the reagents needed to accomplish the following transformation. If more than one…
A: Kindly repost your questions other than (1)
Q: 19. A titration analysis of a 345 mL aqueous HBr solution was performed and the endpoint was reached…
A: Potassium hydroxide reacts with hydrobromic acid, to form potassium bromide and water. The equation…
Q: What volume of 2.3 M solution of NaCl is needed to deliver 1.0 gram of sodium ion?
A:
Q: 2.4 Sketch a generic mechanism for a good nucleophile (Nu:) reacting with R₂C=O. A charged…
A:
Q: QUESTION 1 The Scientific Method is a universal system for performing experimental research. •…
A: Please find the below attachment.
Q: assuming that charcoal and sugar are the main impurities in a sample of crude acetanilide explain…
A: water is a good solvent for the purification and separates all the components of the mixture of…
Q: Explain which is the stronger acid between the following pairs. [Hint: Draw plausible Lewis…
A: Here we have to determine stronger acid among the following pair of acids.
Q: 20 Separation of a Mixture lanens?) 4) A mixture weighing 28.516 g contained 4.518 g NH4C1, 15.250 g…
A:
Q: Determine the molarity for the following solution: 1.8 × 104 mg of HCl in 0.075 L of solution.
A:
Q: Task 3.1. Calculate the standard enthalpy of reaction of some thermochemical equations. Direction:…
A:
Q: Aldehydes are generally more reactive than ketones in nucleophilic addition to the carbonyl group.…
A:
Q: the reaction, N2(g) + O2(g) → 2 NO(g) The rate of disappearance of N2 is 0.887 M/min. What is the…
A:
Q: Repeat the calculation for the value of the K in Part 1b, only this time, do not subtract the [H3O+]…
A: Concept based on the acid dissociation constant of weak acids and finding out the acid dissociation…
Q: Calculate the standard free energy change (kJ/mol) for the reaction shown at a temperature of 298.15…
A: According to the question we have a reaction between the nitrogen gas and oxygen gas which is as…
Q: Sodium phosphate and calcium chloride react to form cal cium phosphate and sodium chloride.
A: Given reaction is double displacement reaction. Double Displacement reaction-: when reaction in…
Q: Fill in the name and empirical formula of each ionic compound that could be formed from the ions in…
A: Here we have to write the empirical formula of the following given ionic compounds obtained from the…
Q: Starting with the chair conformer shown on the left, provide a potential energy plot for the…
A:
Q: The following reaction combines concepts from ch 18 and ch 16. Draw a detailed mechanism for this…
A: The reaction of iodobenzene with a strong base(NaNH2) produces benzyne intermediate. The benzene…
Q: Mechanism. Provide the complete mechanism for the reaction below. You must include appropriate…
A:
Q: Macmillan Learning Combining 0.244 mol Fe₂O3 with excess carbon produced 11.1 g Fe. Fe₂O3 +3C2Fe + 3…
A:
Q: Draw the conformer that would form after the back carbon of this conformer is rotated 60° rotation…
A: The Newman projection represents the dihedral angles of different conformations of a molecule. In…
Q: 1. Question 1 Show the chemical reaction that forms a polyester made of maleic acid and propylene…
A: Reaction of Maleic acid and propylene glycol
Q: Draw out a reaction for 2-chloro-2-methylbutane and KOH, naming each product (if more than one)
A:
Q: Fill in the name and empirical formula of each ionic compound that could be formed from the ions in…
A: Here various cations and anions are given so we can compute the empirical formula just by writing…
Q: In the reaction, 2 N2(g) + 3 O2(g) → 2 N2O3(g) The rate of disappearance of N2 is 0.362 M/min.…
A:
Q: how to balance equation
A: we have to balance the given equations
Q: Complete the table below by writing the symbols for the cation and anion that make up each ionic…
A:
Q: 13) Which is the conjugate acid of the following molecule? a) OH OH b) -OH c) H d) e)
A:
Q: Select the product of the chemical transformation seen below: H₂ Pd (cat.)
A: Given reaction is reduction reaction of alkyne and alkene with H2/pd.
Q: Consider the table below for the reaction, What is the average rate of reaction (mol/L min) over…
A:
Q: 44. Which of the following elements would be expected to show the similar chemical properties based…
A: Periodicity :- In periodic table elements with similar properties repeats in regular intervals. This…
Q: 23 An oxygen atom has a mass of 2.66 x 10 g and a glass of water has a mass of 0.050 kg. Use this…
A: We’ll apply mole concept to Solve the above question .
Q: This is the chemical formula for methyl tert-butyl ether (the clean-fuel gasoline additive MTBE):…
A:
Q: What is the IUPAC name for the compound shown? НО 0 О ОН
A:
Q: Use the rules (in order) to assign oxidation numbers to each of the elements in the compounds below.…
A: Given compounds: Zn(OH)42- HF Cd(OH)2 We have to find the oxidation numbers of each elements in…
Q: Draw the detailed mechanism and predict the major product of each of the following reactions. O₂N.…
A:
Q: In a electrolytic cell, Electrical energy is converted to chemical energy in a non - spontaneous…
A: we have to select the correct statement for an electrolytic cell
Q: The thermal decomposition of N₂O5 obeys first-order kinetics. At 45°C, a plot of In[N₂Og] versus r…
A: First-order reaction: The rate of the first-order reaction depends only on one reactant. According…
Q: For a certain first-order reaction with the general form aA → products, the rate is 0.32 M-s -1 when…
A:
answer 4 please
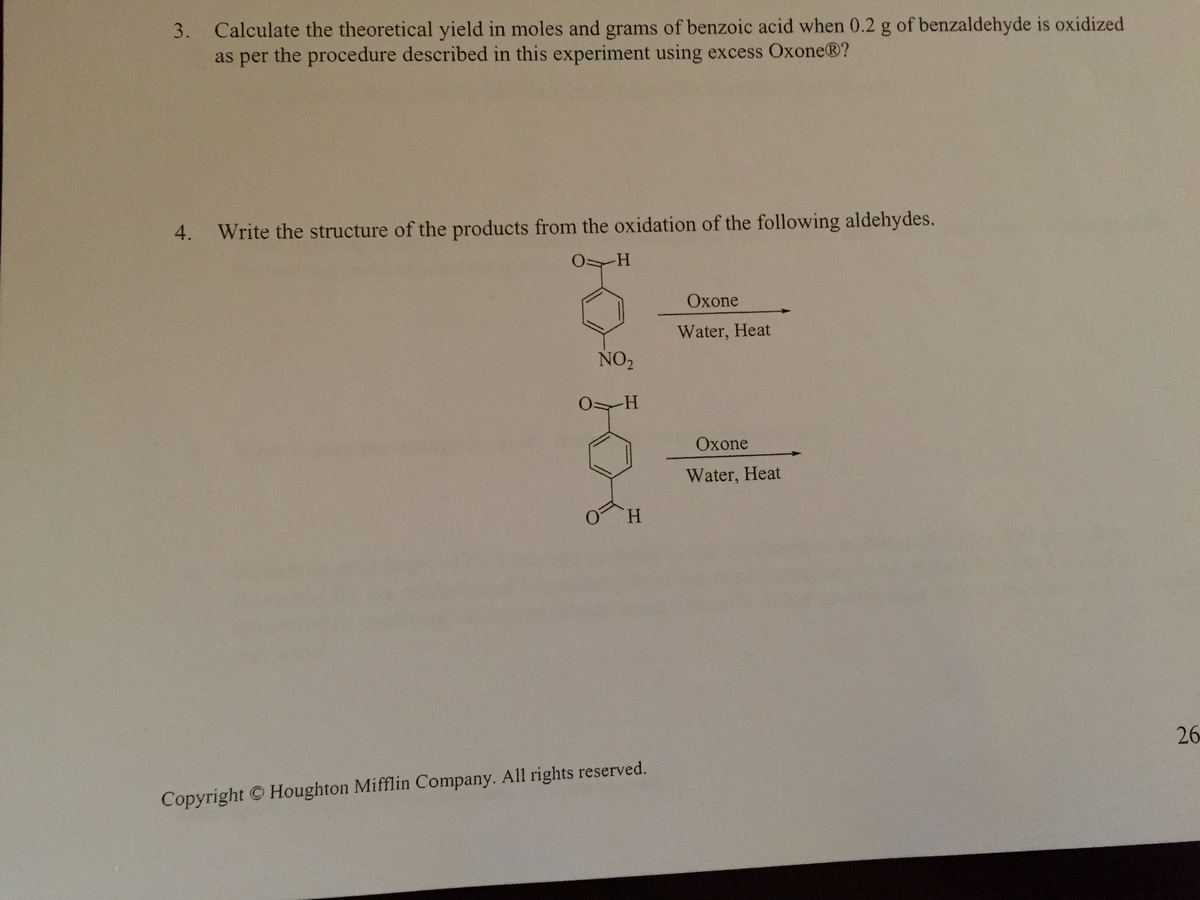
Step by step
Solved in 2 steps with 1 images

- An organic chemistry laboratory Extraction; the extraction of crude naphtalene- benzoic acid mixture. Naphthalene is a suspect carcinogen and is toxic to aquatic life. Find an alternative pair of molecules that adhere better to the principles of green chemistry and can be separated by extraction using green' solvents. (kindly answer fully)Restate the paragraph. The main objective of the laboratory activity was to make aspirin also known as acetylsalicylic acid in its scientific name. It was successfully done as aspirin was synthesized using the combination of salicylic acid and acetic anhydride. In this reaction, salicylic acid's hydroxyl group on the benzene ring interacted with acetic anhydride to generate an ester functional group. This is an esterification reaction that produces acetylsalicylic acid. Sulfuric acid was utilized as a catalyst to start the esterification reaction. Some unreacted acetic anhydride and salicylic acid, as well as sulfuric acid, aspirin, and acetic acid, remained in the solution after the reaction was completed. The process of crystallization was used to obtain pure crystals of a substance from an impure mixture. Upon completion of the lab, analysis, and calculations, , it is evident that the synthesis of aspirin is possible using these methods but that the yield will be relatively lowWhy is diethyl ether used as a solvent for the reduction reaction of the keystone (3,3-dimethyl-2-butanone) utilizing the reducing agent sodium borohydride (NaBH4). what is the purpose of was repeated washes with water? What is the purpose of repeated washes with brine?
- a reaction was preformed in which 0.550 mL of p-anisaldehyde was reacted with a slight excess of benzyltriphenylphosphonium chloride to make 0.646 g of p-methyoxystilbene. calculate the theoretical yield and percent yield for this reaction. 388.9 g/mol benyzyltriphenylphosphonium chloride 136.2 g/mol anisaldehyde d= 1.12 g/mL 210.1 g/mol p-methoxystilbenePls solve this question correctly in 5 min i will give u like for sure Balance the chemical equation of a reaction formed by the bromination of trans-cinnamic acid if we started with 0.3088 grams of trans cinnamic acid and 1mL of bromine. The product of this reaction is 2,3 - dibromo - 3- phenylpropanoic acidWhat is the experimental yield of CRUDE PRODUCT AND FINAL PRODUCT (grams and percentage) Experiment: Oxidation of -Chlorotoluene to o-Chlorobenzoic acid materials used dissolved 3.35 g of KMnO4 1.20 mL of 2-chlorotoluene was added to the mixture 3.0 mL of concentrated hydrochloric acid (pH~2) 10 mL of toluene -The crystals were isolated by vacuum filtration- Product Characterization 1.15 g of the crude product 0.93 g of the final product M.p. (final): 139-141 celsius
- TLC, a powerful analytical tool, can be used to monitor the progress of reactions. The synthesis of ethyl-3-coumarincarboxylate can be monitored by TLC by displaying the starting aldehyde 1, and coumarin product 2, which have very distinct Rf values (Hint: Think about the polarity of compounds 1 and 2 in terms of their abilities to H-bonds to silica gel). What can be determine about the progress of the reaction from analysis of the TLC shown below?In this lab you used acid/base extraction to remove acidic impurities, how could basic impurities be removed through extraction? Imagine the two compounds below are both dissolved in ethyl acetate (a standard organic solvent for extractions), create a diagram/flow chart that outlines steps that could be taken to separate them through acid/base extraction and recover each compound in its neutral Be sure to track when each compound is in the organic or aqueous layer.a) Write down the products that will occur when you extract HBr from 2-bromo-3-methyl butane in a basic medium, State the reaction conditions. Show which product is the main product. b) Does the main product show the geometric isomer, so please write together. If it shows write the isomers. c) Write the product that will be formed when the main product reacts with KMnO4 in a basic environment in cold
- Write the Method used in recrystallisation of benzoic acid, azobenzene, and p-nitroaniline.Write the reaction involved in Ferrox Test. a. What is the species responsible? b. Why is phenol negative in Ferrox Test? Based on the theoretical result, what is the order of reactivity of primary, secondary, and tertiary alcohols in the Lucas Test? a. Lucas Reagent contains ZnCl2 in HCl. What is the role of ZnCl2? What reagents are used in the esterification of Alcohols and Phenols? a. Write the reaction involved in Primary Alcohol (Ethanol) and Acetyl Chloride b. Write the reaction involved in Phenol and Acetyl Chloride What is the purpose of the Chromic acid test? a. What are the reagents used? b. Write oxidation reaction of Primary Alcohols and Secondary AlcoholsA wittig reaction experiment: Week 1 -》 preparation of phosphonium salt Materials : triphenylphosphine (5.3g),methyl Bromoacetate 3.36g (2.1ml) , ethanol 30ml . The yield obtained was 8.93g . Calculate the percentage yield Week 2 -》 Formation of the tlide and wittig reaction Materials :Phosphonium salt (5g), napthyl-2- carboxwaldehyde( 2.65g), 20 ml of water and 5 nl of Nahco3. Yield obtained was 0.51g .Calculate the percentage yield Week 3: Solvent free wittig reaction Materials: Benzyltriphenylphosphonium chloride (0.5g ), 4 - Bromoabenzaldehyde (0.24g), Potassium phosphate (tribasi c) 0.275g .The yield obtained was 1.21g. Calculate the percentage yield

