4. You are to choose the members of an expedition that will climb several high mountains. Each applicant for one of the positions is a heterozygote for an abnormal hemoglobin: Person A: Hb Albany: a methionine has been substituted for Lys 82 in the ß-subunit. Person B: Hb Cowtown: a leucine has been substituted for His 146 in the ß-subunit. Person C: Hb Flagstaff: an isoleucine has been substituted for Val 1 in the ß-subunit. For the locations of the amino acid residues refer to your text, the figure on page 9 of this lab, and the KiNG exercises. Assuming that each of these candidates is equal in ability at low altitude, which one would you choose for the expedition? To answer this question, determine how the substituted amino acid affects the structure and function of each abnormal hemoglobin, and describe the physiological effect. Focus on the stabilization of the T versus R state of hemoglobin, and the consequent physiological changes that might occur at high altitude. Keep your answers brief. Person A Person B Person C
4. You are to choose the members of an expedition that will climb several high mountains. Each applicant for one of the positions is a heterozygote for an abnormal hemoglobin: Person A: Hb Albany: a methionine has been substituted for Lys 82 in the ß-subunit. Person B: Hb Cowtown: a leucine has been substituted for His 146 in the ß-subunit. Person C: Hb Flagstaff: an isoleucine has been substituted for Val 1 in the ß-subunit. For the locations of the amino acid residues refer to your text, the figure on page 9 of this lab, and the KiNG exercises. Assuming that each of these candidates is equal in ability at low altitude, which one would you choose for the expedition? To answer this question, determine how the substituted amino acid affects the structure and function of each abnormal hemoglobin, and describe the physiological effect. Focus on the stabilization of the T versus R state of hemoglobin, and the consequent physiological changes that might occur at high altitude. Keep your answers brief. Person A Person B Person C
Biology: The Dynamic Science (MindTap Course List)
4th Edition
ISBN:9781305389892
Author:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:Peter J. Russell, Paul E. Hertz, Beverly McMillan
Chapter15: From Dna To Protein
Section: Chapter Questions
Problem 4TYK
Related questions
Question

Transcribed Image Text:4. You are to choose the members of an expedition that will climb several high mountains.
Each applicant for one of the positions is a heterozygote for an abnormal hemoglobin:
Person A: Hb Albany: a methionine has been substituted for Lys 82 in the B-subunit.
Person B: Hb Cowtown: a leucine has been substituted for His 146 in the B-subunit.
Person C: Hb Flagstaff: an isoleucine has been substituted for Val 1 in the B-subunit.
For the locations of the amino acid residues refer to your text, the figure on page 9 of this lab,
and the KING exercises. Assuming that each of these candidates is equal in ability at low
altitude, which one would you choose for the expedition? To answer this question, determine
how the substituted amino acid affects the structure and function of each abnormal
hemoglobin, and describe the physiological effect. Focus on the stabilization of the T versus
R state of hemoglobin, and the consequent physiological changes that might occur
altitude. Keep your answers brief.
high
Person A
Person B
Person C
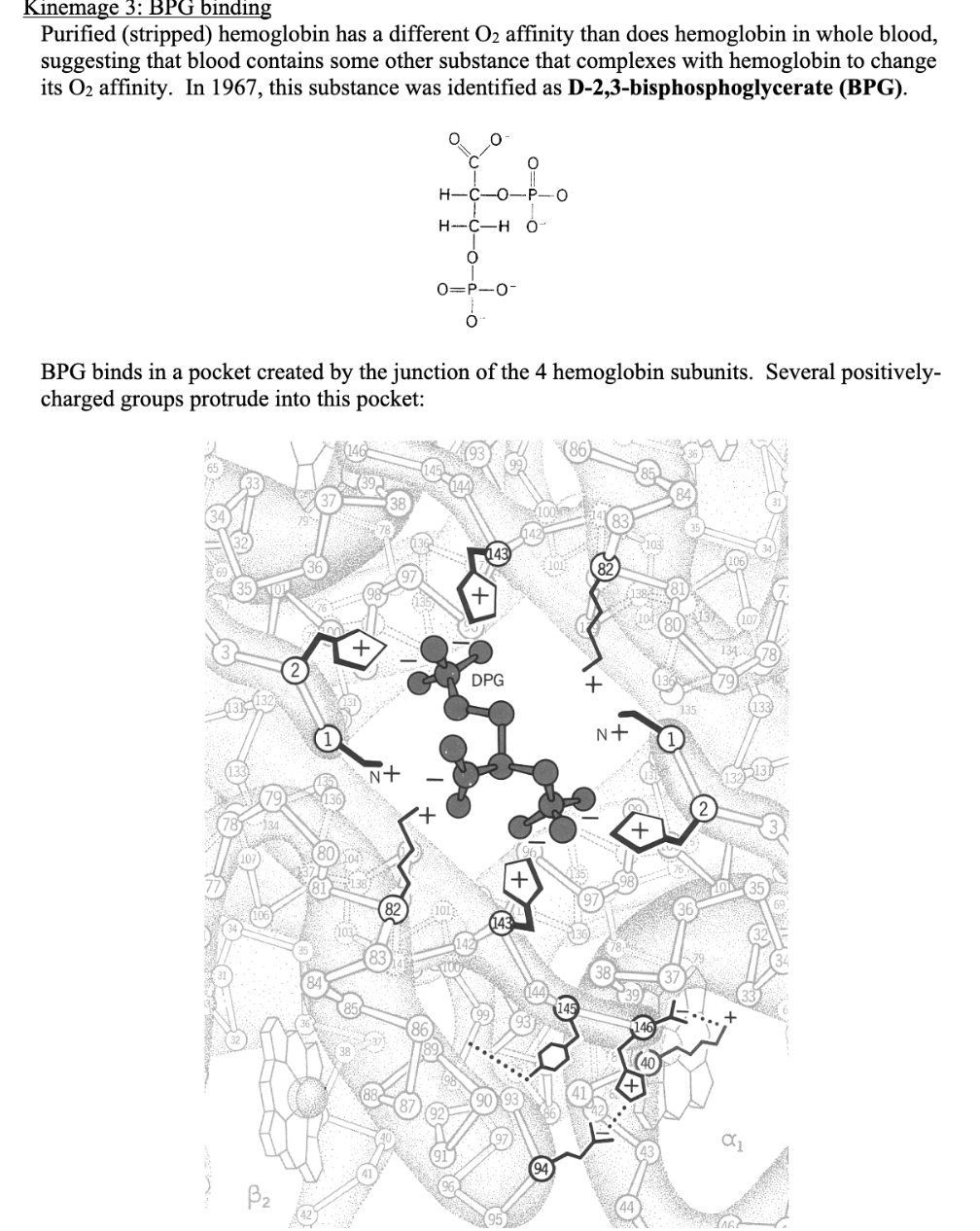
Transcribed Image Text:Kinemage 3: BPG binding
Purified (stripped) hemoglobin has a different O2 affinity than does hemoglobin in whole blood,
suggesting that blood contains some other substance that complexes with hemoglobin to change
its O2 affinity. In 1967, this substance was identified as D-2,3-bisphosphoglycerate (BPG).
H-C-0-P-0
H-C-H Ó
0=P-0-
BPG binds in a pocket created by the junction of the 4 hemoglobin subunits. Several positively-
charged groups protrude into this pocket:
83
P103
143
82.
78
DPG
N+
n+
643
B2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning

Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:
9781337392938
Author:
Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:
Cengage Learning