Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.37E
Related questions
Question
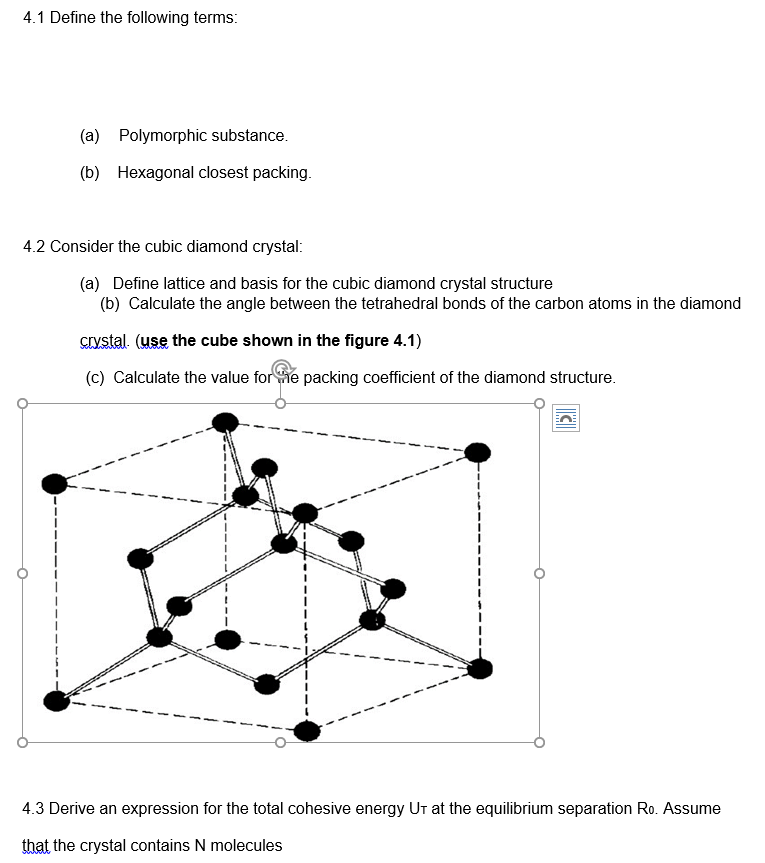
Transcribed Image Text:4.1 Define the following terms:
(a) Polymorphic substance.
(b) Hexagonal closest packing.
4.2 Consider the cubic diamond crystal:
(a) Define lattice and basis for the cubic diamond crystal structure
(b) Calculate the angle between the tetrahedral bonds of the carbon atoms in the diamond
crystal. (use the cube shown in the figure 4.1)
(c) Calculate the value for the packing coefficient of the diamond structure.
4.3 Derive an expression for the total cohesive energy UT at the equilibrium separation Ro. Assume
that the crystal contains N molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning