4.The diagram below is an example of Two Perfectly Miscible Liquids (T vs Mole Fraction).Fill out the question marks with independent components and degrees of freedom. correct number of phases,number of
4.The diagram below is an example of Two Perfectly Miscible Liquids (T vs Mole Fraction).Fill out the question marks with independent components and degrees of freedom. correct number of phases,number of
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter6: Steam Distillation, Vacuum Distillation, And Sublimation
Section: Chapter Questions
Problem 6Q: A mixture of toluene (bp110.8C) and water is steam distilled. Visual inspection of the distillate...
Related questions
Question
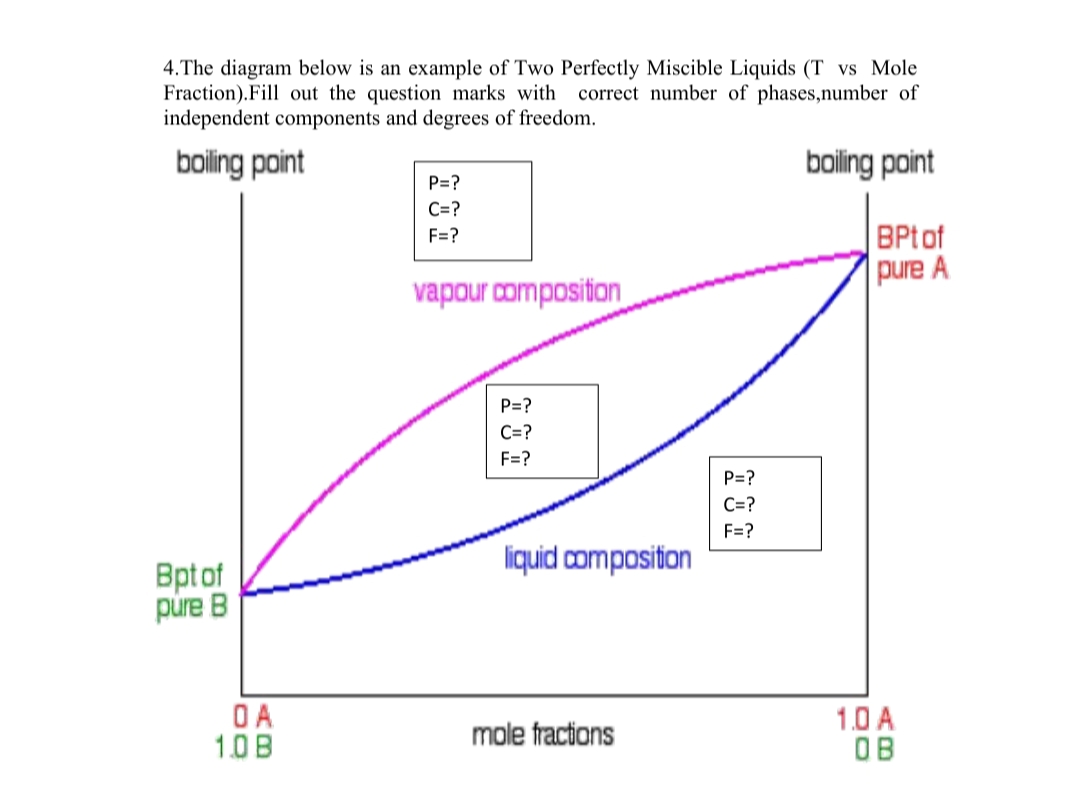
Transcribed Image Text:4.The diagram below is an example of Two Perfectly Miscible Liquids (T vs Mole
Fraction).Fill out the question marks with correct number of phases,number of
independent components and degrees of freedom.
boiling point
boling point
P=?
C=?
F=?
BPtof
pure A
vapour composition
P=?
C=?
F=?
P=?
C=?
F=?
liquid composition
Bpt of
pure B
OA
1.0B
1.0 A
OB
mole fractions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning