4000 100 80 60 40 IR Spectrum (liquid film) % of base peak C-A 3000 43 10 40 13C NMR Spectrum (100 MHz, CDCI, solution) DEPT CH, CH, CH M = 86 80 proton decoupled 200 ¹H NMR Spectrum (200 MHz, CDCI, solution) 9 8 ww 1716 1200 800 1600 V (cm¹) Mass Spectrum 2000 120 160 m/e 160 7 200 120 6 240 C4H602 280 5 solvent 80 4 Problem 6 حلتك او " بلون 2 UV Spectrum max 289 nm (log, 1.4) solvent methanol گرد 40 3 200 3 2-4 2 112 8 (ppm) TI 1
4000 100 80 60 40 IR Spectrum (liquid film) % of base peak C-A 3000 43 10 40 13C NMR Spectrum (100 MHz, CDCI, solution) DEPT CH, CH, CH M = 86 80 proton decoupled 200 ¹H NMR Spectrum (200 MHz, CDCI, solution) 9 8 ww 1716 1200 800 1600 V (cm¹) Mass Spectrum 2000 120 160 m/e 160 7 200 120 6 240 C4H602 280 5 solvent 80 4 Problem 6 حلتك او " بلون 2 UV Spectrum max 289 nm (log, 1.4) solvent methanol گرد 40 3 200 3 2-4 2 112 8 (ppm) TI 1
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter20: Molecular Mass Spectrometry
Section: Chapter Questions
Problem 20.11QAP
Related questions
Question
What is the IR and HNMRAndCNMR and mass in this example explain the chemical shift
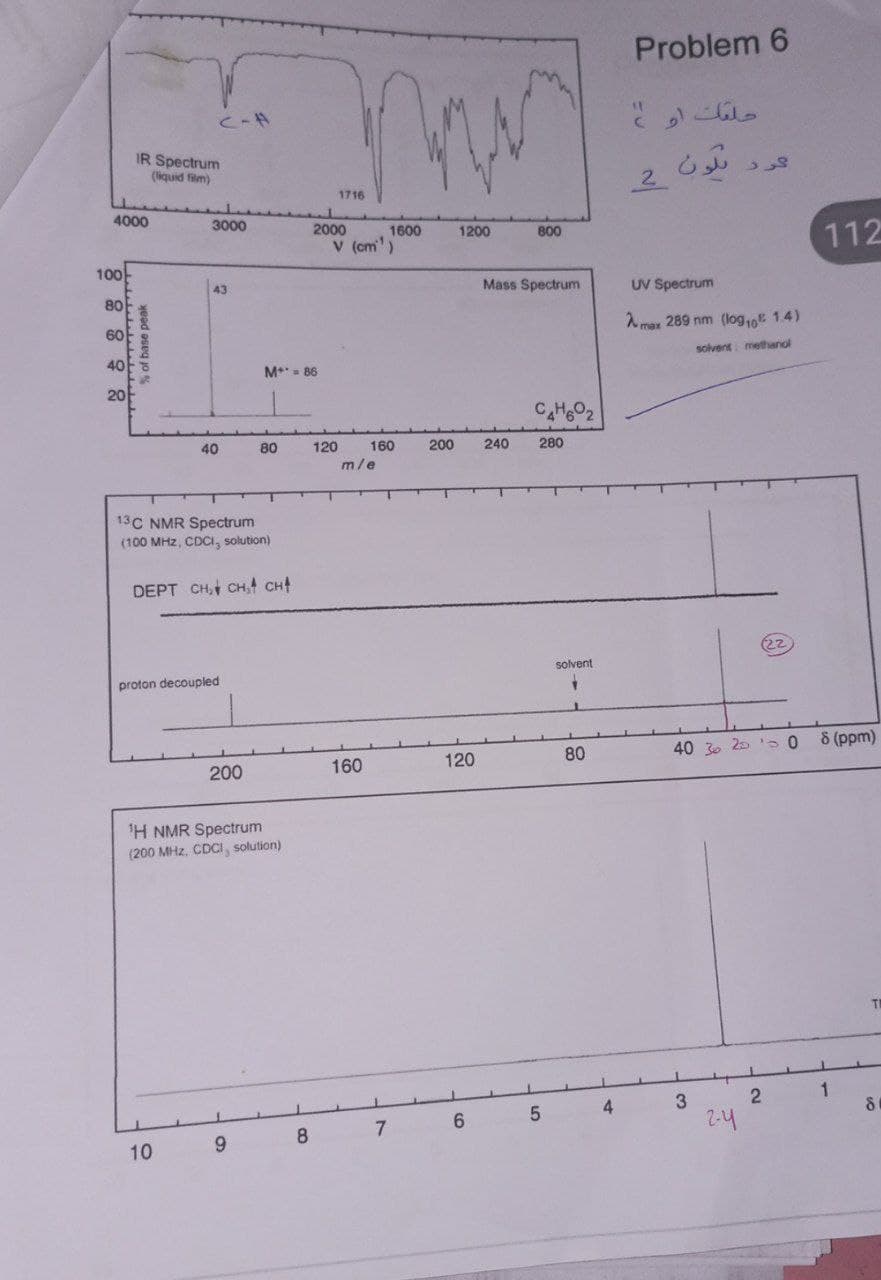
Transcribed Image Text:4000
100
IR Spectrum
(liquid film)
80
60
% of base peak
C-A
3000
43
10
40
13C NMR Spectrum
(100 MHz, CDCI, solution)
DEPT CH₂ CH₂ CH4
M+* = 86
80
proton decoupled
200
¹H NMR Spectrum
(200 MHz, CDCI, solution)
9
8
m
1716
1200
1600
V (cm¹)
2000
120
160
m/e
160
7
200 240
120
800
Mass Spectrum
6
C4H602
280
5
solvent
+
80
4
Problem 6
حلقات او )
لون
2
UV Spectrum
max 289 nm (log, 1.4)
solvent methanol
40 30 20
2
1
3
کرد
2-4
112
8 (ppm)
8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning