The molecules below is Testosterone, which is the male sex hormone. OH CH3 CH3 Which of the following functional groups does testosterone contain? (a) Carbonyl (b) Ester (c) Carboxylic acid (d) Amine Which of the reactions between these pairs of compounds or reagents is an addition reaction? (a) ethane + chlorine (b) ethene + bromine (c) ethanol + hydrogen bromide (d) bromoethane + aqueous sodium hydroxide Which of the following is not a Lewis base? (a) NH3 (b) H- (c) (d) BF3 H₂O
The molecules below is Testosterone, which is the male sex hormone. OH CH3 CH3 Which of the following functional groups does testosterone contain? (a) Carbonyl (b) Ester (c) Carboxylic acid (d) Amine Which of the reactions between these pairs of compounds or reagents is an addition reaction? (a) ethane + chlorine (b) ethene + bromine (c) ethanol + hydrogen bromide (d) bromoethane + aqueous sodium hydroxide Which of the following is not a Lewis base? (a) NH3 (b) H- (c) (d) BF3 H₂O
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter22: Organic Chemistry And Biochemistry
Section: Chapter Questions
Problem 88QE
Related questions
Question
Please answer all
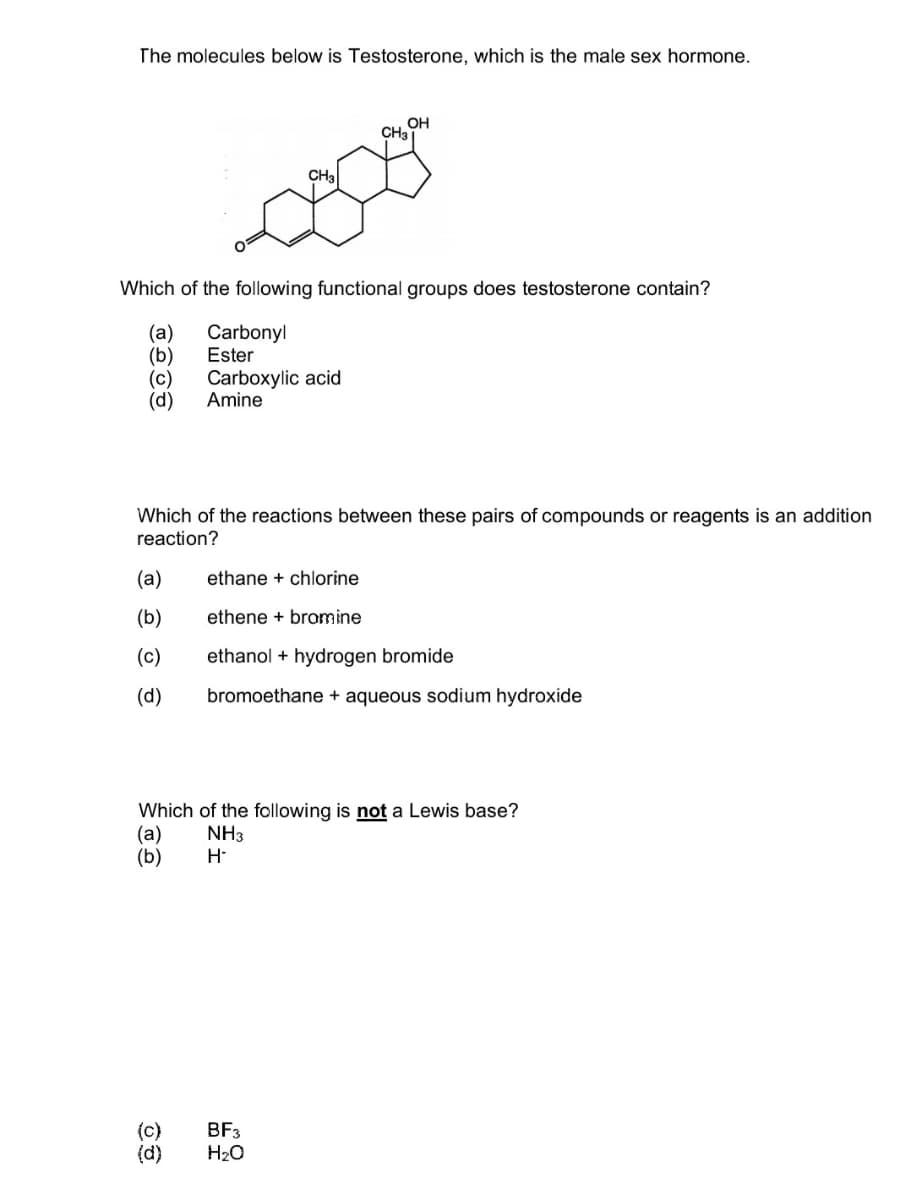
Transcribed Image Text:The molecules below is Testosterone, which is the male sex hormone.
OH
CH3
CH₂
Which of the following functional groups does testosterone contain?
(a)
Carbonyl
(b)
Ester
(c) Carboxylic acid
(d) Amine
Which of the reactions between these pairs of compounds or reagents is an addition
reaction?
(a)
ethane + chlorine
(b)
ethene + bromine
(c)
ethanol + hydrogen bromide
(d)
bromoethane + aqueous sodium hydroxide
Which of the following is not a Lewis base?
(a) NH3
(b)
(c)
(d)
H-
BF3
H₂O

Transcribed Image Text:The pH of a 0.1 mol dm³ solution of a weak acid with a Ka = 10.⁹ will be...
(a)
2.5
(b)
5
(c)
10
(d)
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning