4/18 tasks attempted he Haber-Bosch process involves the combination of nitrogen and hydrogen at high temperatures in the resence of a catalyst to produce ammonia. It is currently the main industrial source of ammonia. N2 (g) +3 H2 (g) →2 NH3 (g) Assuming that a reaction vessel originally contains 0.400 moles of N2 and 0.900 moles of H2, answer each of the following questions: a) How many moles of NH3 could be produced from these quantities of N2 and H2 ? NH3 mol b) After the reaction is complete, how many moles of N2 would remain? N2 mol c) After the reaction is complete, how many moles of H2 would remain? H2 moles SAVE RESPONSE 1. 6 7 18 PRIVACY POLICY TERMS OF USE CONTACT INFORMATION 5.
4/18 tasks attempted he Haber-Bosch process involves the combination of nitrogen and hydrogen at high temperatures in the resence of a catalyst to produce ammonia. It is currently the main industrial source of ammonia. N2 (g) +3 H2 (g) →2 NH3 (g) Assuming that a reaction vessel originally contains 0.400 moles of N2 and 0.900 moles of H2, answer each of the following questions: a) How many moles of NH3 could be produced from these quantities of N2 and H2 ? NH3 mol b) After the reaction is complete, how many moles of N2 would remain? N2 mol c) After the reaction is complete, how many moles of H2 would remain? H2 moles SAVE RESPONSE 1. 6 7 18 PRIVACY POLICY TERMS OF USE CONTACT INFORMATION 5.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.77PAE: The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce...
Related questions
Question
Need help
Solving this
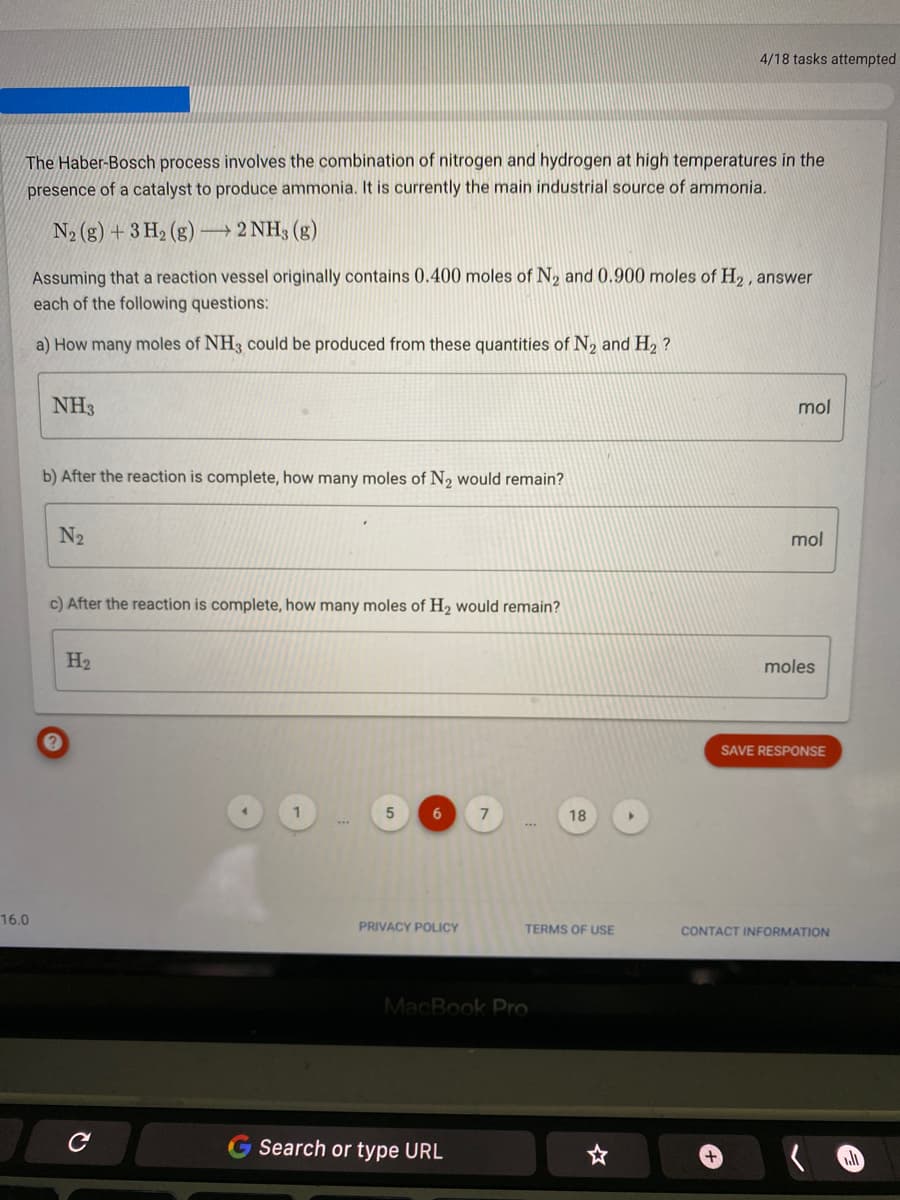
Transcribed Image Text:4/18 tasks attempted
The Haber-Bosch process involves the combination of nitrogen and hydrogen at high temperatures in the
presence of a catalyst to produce ammonia. It is currently the main industrial source of ammonia.
N2 (g) + 3 H2 (g) 2 NH3 (g)
Assuming that a reaction vessel originally contains 0.400 moles of N, and 0.900 moles of H,, answer
each of the following questions:
a) How many moles of NH3 could be produced from these quantities of N, and H2 ?
NH3
mol
b) After the reaction is complete, how many moles of N2 would remain?
N2
mol
c) After the reaction is complete, how many moles of H2 would remain?
H2
moles
SAVE RESPONSE
6.
18
16.0
PRIVACY POLICY
TERMS OF USE
CONTACT INFORMATION
MacBook Pro
Search or type URL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning