4:39 PM Wed Feb 12 A 73% chem 1b readings chem 1b readings chem 1B discussion ఈ (C) (D) (E) He extrapolated to the point at which the pressure would be zero He extrapolated to the point at which the apparent number of moles would be zero none of these 20.188/mol 20.18g Jvey \యం { (3) Suppose a gas mixture, containing Ne and Ar gases, is maintained at a temperature where the most probable speed for Ne atoms is 201.80 m/s. The most probable speed for Ar atoms is )(32.448) :143. 42 03995 (A) 201.8 m/sec (B) 399.48 m/sec. (C) 101.94 m/sec. (D) 143.43 m/sec. (E) none of these 3 18 0.0 (4) Krypton, Kr, gas of mass 3.900 gram reacts with fluorine, F, to form 5.670 gram of a krypton fluoride. What is the empirical formula for this compound? 0.82013 24.94- 0.08 (A) KrF (B) KrF2. (C) KrF4. (D) KrF6. (E) KrF8 (5) A plot of the relative number of gas particles versus speed is shown for two gas samples: 500 Speed 1000 1500 2000 шetenseс Relative Number
4:39 PM Wed Feb 12 A 73% chem 1b readings chem 1b readings chem 1B discussion ఈ (C) (D) (E) He extrapolated to the point at which the pressure would be zero He extrapolated to the point at which the apparent number of moles would be zero none of these 20.188/mol 20.18g Jvey \యం { (3) Suppose a gas mixture, containing Ne and Ar gases, is maintained at a temperature where the most probable speed for Ne atoms is 201.80 m/s. The most probable speed for Ar atoms is )(32.448) :143. 42 03995 (A) 201.8 m/sec (B) 399.48 m/sec. (C) 101.94 m/sec. (D) 143.43 m/sec. (E) none of these 3 18 0.0 (4) Krypton, Kr, gas of mass 3.900 gram reacts with fluorine, F, to form 5.670 gram of a krypton fluoride. What is the empirical formula for this compound? 0.82013 24.94- 0.08 (A) KrF (B) KrF2. (C) KrF4. (D) KrF6. (E) KrF8 (5) A plot of the relative number of gas particles versus speed is shown for two gas samples: 500 Speed 1000 1500 2000 шetenseс Relative Number
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.119P
Related questions
Question
I'm unsure of how to answer the question attached below the question is highlighted in red.
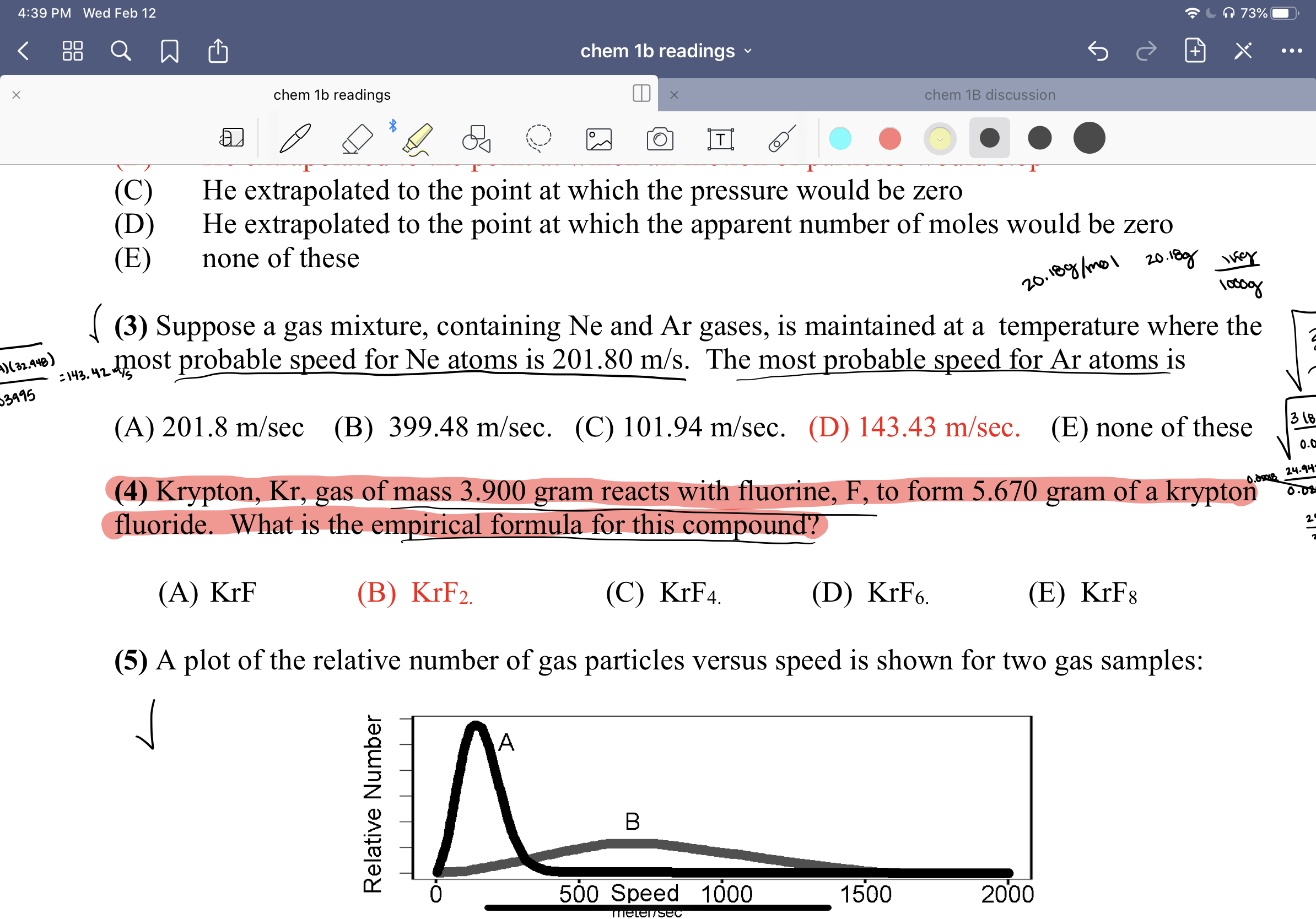
Transcribed Image Text:4:39 PM Wed Feb 12
A 73%
chem 1b readings
chem 1b readings
chem 1B discussion
ఈ
(C)
(D)
(E)
He extrapolated to the point at which the pressure would be zero
He extrapolated to the point at which the apparent number of moles would be zero
none of these
20.188/mol 20.18g Jvey
\యం
{ (3) Suppose a gas mixture, containing Ne and Ar gases, is maintained at a temperature where the
most probable speed for Ne atoms is 201.80 m/s. The most probable speed for Ar atoms is
)(32.448)
:143. 42
03995
(A) 201.8 m/sec
(B) 399.48 m/sec. (C) 101.94 m/sec. (D) 143.43 m/sec. (E) none of these
3 18
0.0
(4) Krypton, Kr, gas of mass 3.900 gram reacts with fluorine, F, to form 5.670 gram of a krypton
fluoride. What is the empirical formula for this compound?
0.82013 24.94-
0.08
(A) KrF
(B) KrF2.
(C) KrF4.
(D) KrF6.
(E) KrF8
(5) A plot of the relative number of gas particles versus speed is shown for two gas samples:
500 Speed
1000
1500
2000
шetenseс
Relative Number
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning