4c) is the tricky one must have confused myself and got it wrong but I got A) and B) correct as I will attach my work in a picture below as well thank you.
4c) is the tricky one must have confused myself and got it wrong but I got A) and B) correct as I will attach my work in a picture below as well thank you.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter4: Chemical Reactions
Section: Chapter Questions
Problem 4.70QP
Related questions
Question
4c) is the tricky one must have confused myself and got it wrong but I got A) and B) correct as I will attach my work in a picture below as well thank you.
Transcribed Image Text:Chem 111 > Exams
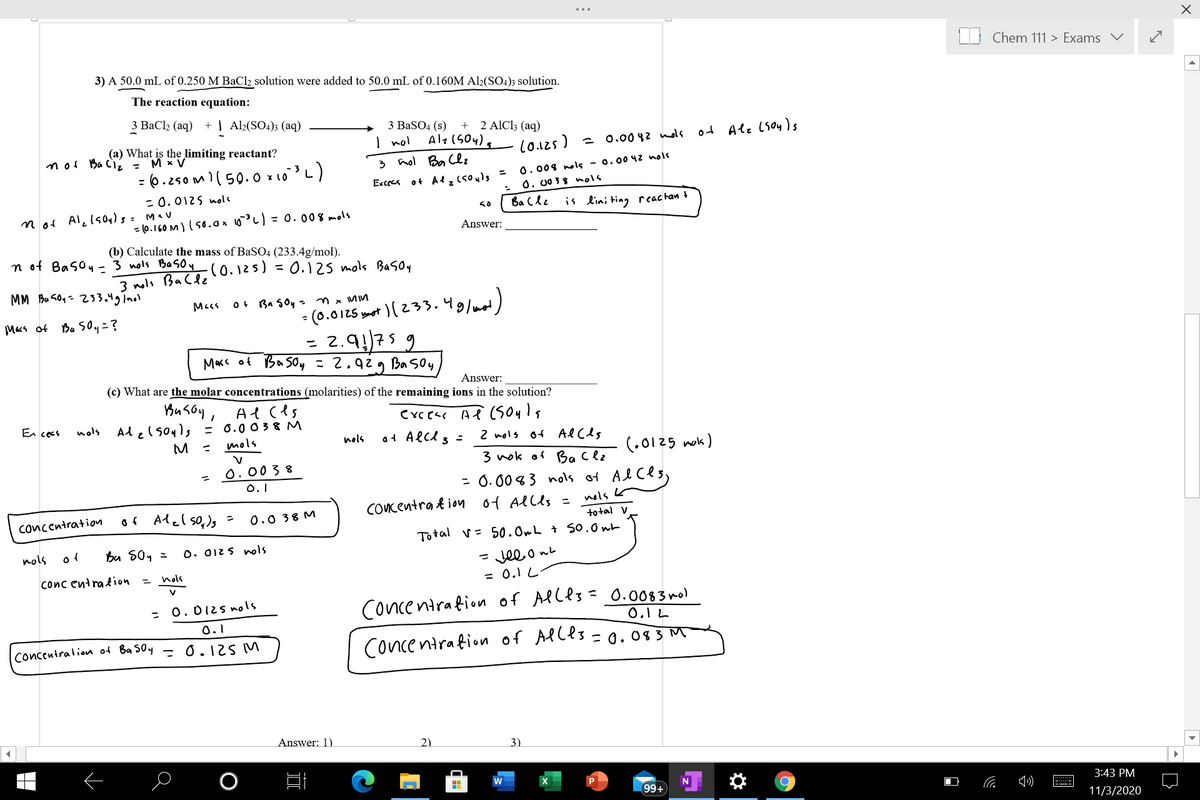
3) A 50.0 mL of 0.250 M BaCl2 solution were added to 50.0 mL of 0.160M Al2(SO4)3 solution.
The reaction equation:
3 ВаСlz (aq)
+ | Al2(SO4)3 (aq)
З BaSO4 (s)
I nol
+ 2 AICI3 (aq)
(a) What is the limiting reactant?
M x V
(0.125)
o4 Ale (soys
n of Ba Ciį =
2 0.00 4z nols
:(6.250m)(50.0 × 10L)
3 nol Ba Clz
Eccres ot Al, csouls =
%3D
0.008 nols - 0.0042 nols
= 0.0125 nola
0. 0038 ols
not Al, lsoy)s= M«v
Ba Cle
is lini ting reactan +
= (0.160 M)( s0.0x 6)= 0. 008 mols
Answer:
(b) Calculate the mass of BaSO4 (233.4g/mol).
n of Basoy - 3 nols Basoy(0.125) = 0.12s mols Basoy
3 nols Bacle
MM Bu soy- 233.4gInol
- (0.0125 yaet ) (233.49/mat)
= 2.9!75 9
Mass of Ba soy = 2.929 Ba soy
Mas
Ba Soy = n
Macs of
Ba Soy =?
(c) What are the molar concentrations (molarities) of the remaining ions in the solution?
Answer:
Al ces
Alelsouls = 0.0038 M
mols
En cecs
nols
o4 Alcl 3 =
2 nols of Alcls
M
nols
(.0125 nok)
3 nok of Ba clz
0.0038
0.1
= 0. 00% 3 nols of Al ces,
nols k
to tal V
Total v= 50.OmL + So.0wt
of Alel so,)s =
COcentra t ion of Allls =
Concentration
0.0 38 M
nols
Bu S0y =
O. 012s ols
- Jee o ne
= 0.1 L
conc entra tion
nols
O. 0I2s nols
0.1
concentration of Ba Soy - 0.125 M
Concentrafion of Alles = 0.0083nol
0.1 L
Concentrafion of Alles = 0.08 3 M
%3D
Answer: 1)
2)
3)
99+
3:43 PM
»)
11/3/2020
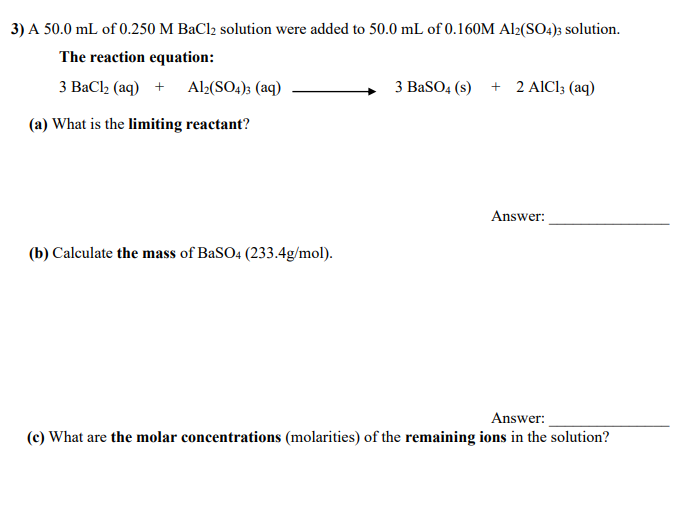
Transcribed Image Text:3) A 50.0 mL of 0.250 M BaCl2 solution were added to 50.0 mL of 0.160M Al2(SO4)3 solution.
The reaction equation:
3 BaCl2 (aq) + Al2(SO4); (aq)
3 BaSO4 (s) + 2 AIC1; (aq)
(a) What is the limiting reactant?
Answer:
(b) Calculate the mass of BaSO4 (233.4g/mol).
Answer:
(c) What are the molar concentrations (molarities) of the remaining ions in the solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning