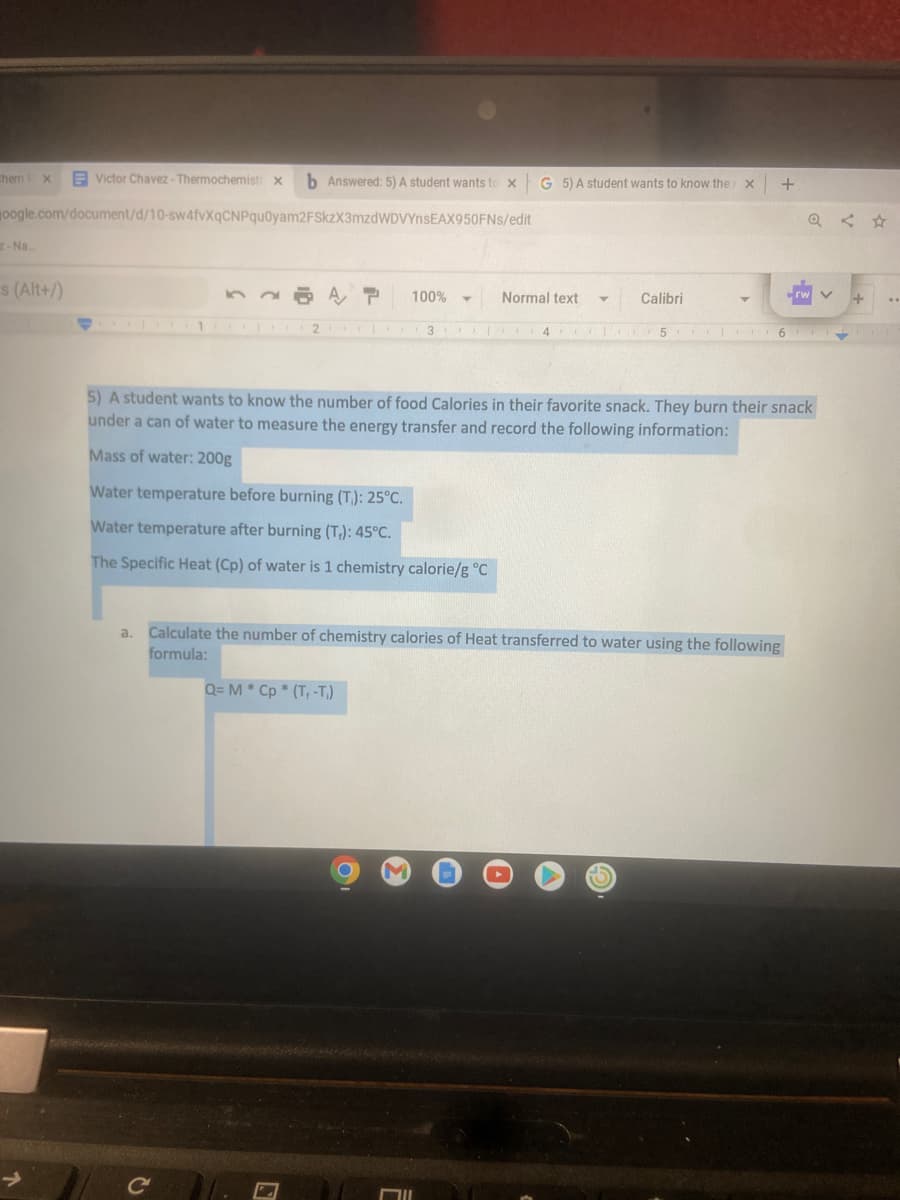
5) A student wants to know the number of food Calories in their favorite snack. They burn their snack under a can of water to measure the energy transfer and record the following information: Mass of water: 200g Water temperature before burning (T): 25°C. Water temperature after burning (T₁): 45°C. The Specific Heat (Cp) of water is 1 chemistry calorie/g °C a. Calculate the number of chemistry calories of Heat transferred to water using the following formula: Q= M * Cp * (T, -1))
5) A student wants to know the number of food Calories in their favorite snack. They burn their snack under a can of water to measure the energy transfer and record the following information: Mass of water: 200g Water temperature before burning (T): 25°C. Water temperature after burning (T₁): 45°C. The Specific Heat (Cp) of water is 1 chemistry calorie/g °C a. Calculate the number of chemistry calories of Heat transferred to water using the following formula: Q= M * Cp * (T, -1))
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 75QAP: Some solar-heated homes use large beds of rocks to store heat. (a) How much heat is absorbed by...
Related questions
Question
Transcribed Image Text:Chem F X
Victor Chavez-Thermochemist x b Answered: 5) A student wants to X
G 5) A student wants to know the x
moogle.com/document/d/10-sw4fvXqCNPqu0yam2FSkzX3mzdWDVYnsEAX950FNs/edit
z-Na...
s (Alt+/)
P
100%
Normal text
Calibri
Y
2
3
4
CO
56
+
5) A student wants to know the number of food Calories in their favorite snack. They burn their snack
under a can of water to measure the energy transfer and record the following information:
Mass of water: 200g
Water temperature before burning (T.): 25°C.
Water temperature after burning (T₁): 45°C.
The Specific Heat (Cp) of water is 1 chemistry calorie/g °C
a. Calculate the number of chemistry calories of Heat transferred to water using the following
formula:
Q= M * Cp * (T+ -T)
TO
וור
+
V
C+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning