5 B. A student writes the following incorrect chemical equation for a single replacement reaction between lithium bromide and fluorine. 2LİBR (aq) + F, (g) a. In a single replacement reaction, part of an ionic compound is removed and replaced by a new element. What element will fluorine actually replace in lithium 2Li (s) + 2FBr (g) bromide?
5 B. A student writes the following incorrect chemical equation for a single replacement reaction between lithium bromide and fluorine. 2LİBR (aq) + F, (g) a. In a single replacement reaction, part of an ionic compound is removed and replaced by a new element. What element will fluorine actually replace in lithium 2Li (s) + 2FBr (g) bromide?
Chapter2: Atoms, Molecules, And Ions
Section: Chapter Questions
Problem 108CWP
Related questions
Question
I need help with these questions please
(Not honor class)
(Not grading)
Transcribed Image Text:-ons Help
Last edit was 8 minutes ago
arial
В I U A
11
= 三
2 3
4
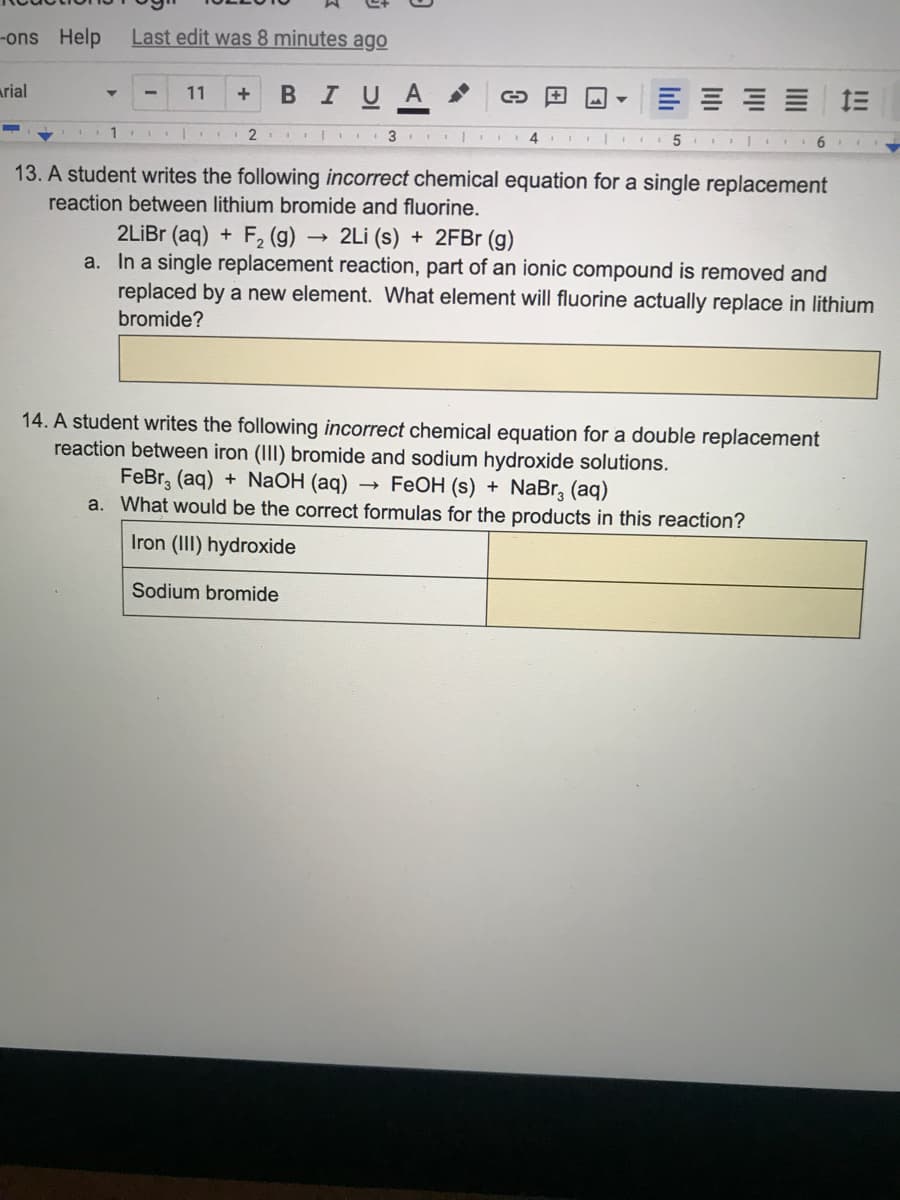
13. A student writes the following incorrect chemical equation for a single replacement
reaction between lithium bromide and fluorine.
2LİBR (aq) + F2 (g) -
a. In a single replacement reaction, part of an ionic compound is removed and
replaced by a new element. What element will fluorine actually replace in lithium
- 2Li (s) + 2FB (g)
bromide?
14. A student writes the following incorrect chemical equation for a double replacement
reaction between iron (III) bromide and sodium hydroxide solutions.
FeBr, (aq) + NaOH (aq) → FeOH (s) + NaBr, (aq)
a. What would be the correct formulas for the products in this reaction?
Iron (III) hydroxide
Sodium bromide
lılı
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning