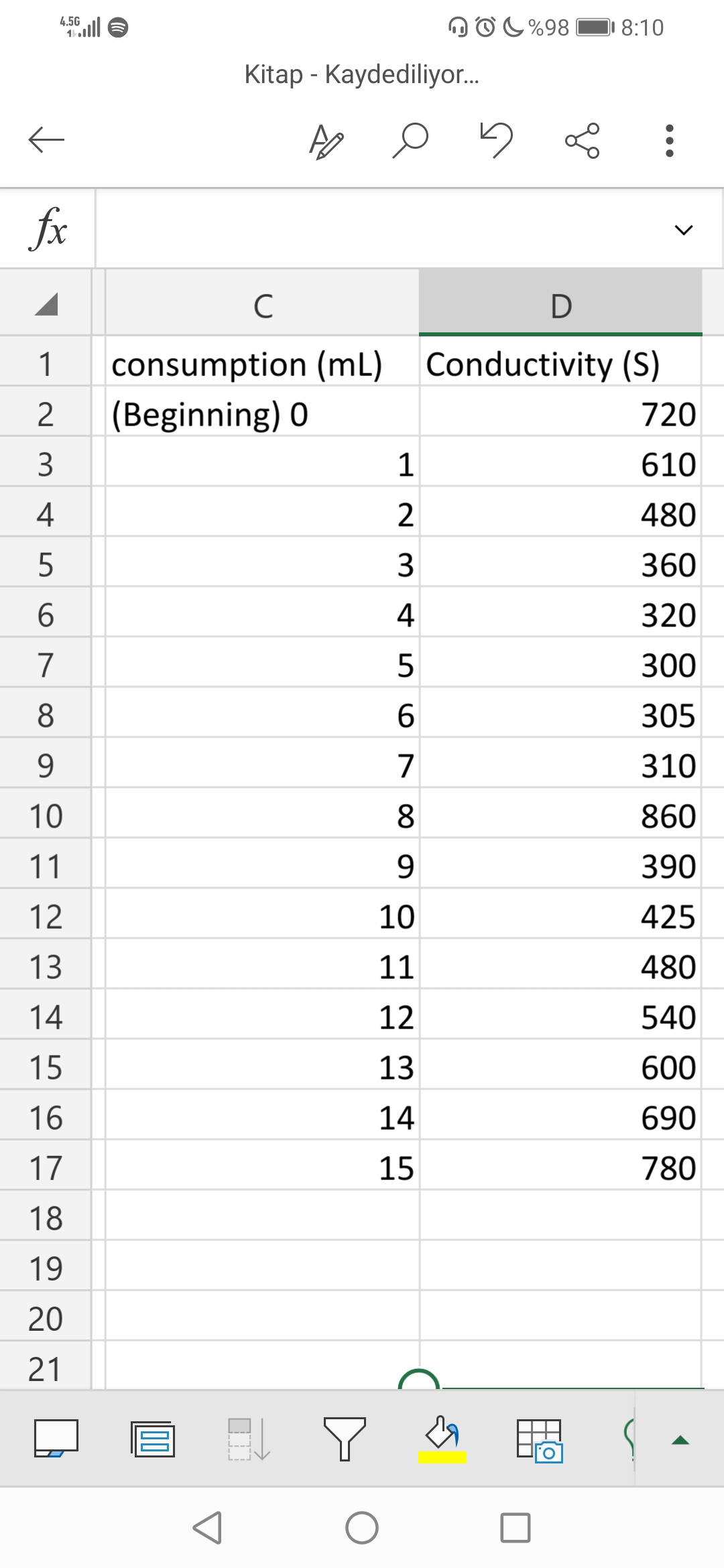
5 mL of HCl and 5 mL of CH3COOH solutions are titrated with a pipette in a glass bottle and 150 mL of distilled water is added. The initial conductivity (720) of this diluted mixture is measured and recorded in the table. Then, one ml of M/10 NaOH solution is added and their conductivity is measured and recorded in the table. Find the concentration of HCl and CH3COOH.
5 mL of HCl and 5 mL of CH3COOH solutions are titrated with a pipette in a glass bottle and 150 mL of distilled water is added. The initial conductivity (720) of this diluted mixture is measured and recorded in the table. Then, one ml of M/10 NaOH solution is added and their conductivity is measured and recorded in the table. Find the concentration of HCl and CH3COOH.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.14QAP
Related questions
Question
5 mL of HCl and 5 mL of CH3COOH solutions are titrated with a pipette in a glass bottle and 150 mL of distilled water is added. The initial conductivity (720) of this diluted mixture is measured and recorded in the table. Then, one ml of M/10 NaOH solution is added and their conductivity is measured and recorded in the table. Find the concentration of HCl and CH3COOH.
Transcribed Image Text:C%98
4.5G
1 8:10
Kitap - Kaydediliyor.
G
fx
C
1
consumption (mL) Conductivity (S)
2
(Beginning) 0
720
3
1
610
4
2
480
360
4
320
7
5
300
8
305
9.
7
310
10
8
860
11
9.
390
12
10
425
13
11
480
14
12
540
15
13
600
16
14
690
17
15
780
18
19
20
21
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning