5. 1 Complete the following reaction scheme and indicate the acid, base, conjugate acid and conjugate base: :NH3 H-O-H :0 5.2 Explain how each, the Lowry-Bronsted and the Lewis definition of an acid and base can be used to identify the acid and base in one or more of the examples from 5.1.
5. 1 Complete the following reaction scheme and indicate the acid, base, conjugate acid and conjugate base: :NH3 H-O-H :0 5.2 Explain how each, the Lowry-Bronsted and the Lewis definition of an acid and base can be used to identify the acid and base in one or more of the examples from 5.1.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.34QAP
Related questions
Question
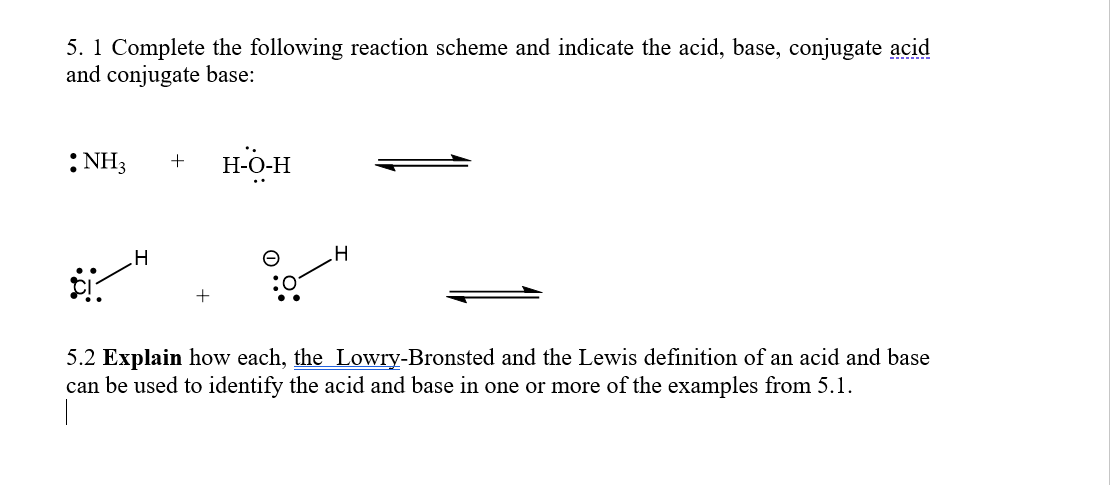
Transcribed Image Text:5. 1 Complete the following reaction scheme and indicate the acid, base, conjugate acid
and conjugate base:
:NH3
H-O-H
+
5.2 Explain how each, the Lowry-Bronsted and the Lewis definition of an acid and base
can be used to identify the acid and base in one or more of the examples from 5.1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning