14. Using the tiny pK, table below, answer these questions for each acid-base question below. |Add на но нро, не сн,сом нсо, нs мне наN CHOH pka -7 -17 22 3.2 4.7 1) Which act as acids? There will be two for every equation – one on each side. 2) Which is the stronger base? There's two to choose from – one on each side. НРо Н,о (снсон Nн, 64 7.0 92 9.3 10.0 12.3 15.7 19 34 3) Which side of the equilibrium is favored? (CH)CОН + он- (CH:)»CO + H;O HF + CH:CO;Na NaF + CH:CO;H H2S + CHsONa NaSH + CHsOH NACN + NazHPO4 NasPO. + HCN H;CO: + NH3 + NaCl NaHCO: + NH.CI
14. Using the tiny pK, table below, answer these questions for each acid-base question below. |Add на но нро, не сн,сом нсо, нs мне наN CHOH pka -7 -17 22 3.2 4.7 1) Which act as acids? There will be two for every equation – one on each side. 2) Which is the stronger base? There's two to choose from – one on each side. НРо Н,о (снсон Nн, 64 7.0 92 9.3 10.0 12.3 15.7 19 34 3) Which side of the equilibrium is favored? (CH)CОН + он- (CH:)»CO + H;O HF + CH:CO;Na NaF + CH:CO;H H2S + CHsONa NaSH + CHsOH NACN + NazHPO4 NasPO. + HCN H;CO: + NH3 + NaCl NaHCO: + NH.CI
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 52A
Related questions
Question
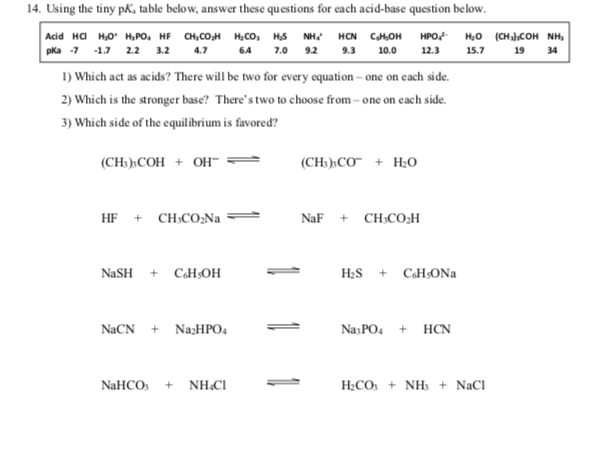
Transcribed Image Text:14. Using the tiny pK, table below, answer these questions for each acid-base question below.
|Add на но нро, не сн,сом нсо, нs мне наN CHOH
pka -7 -17 22 3.2 4.7
1) Which act as acids? There will be two for every equation – one on each side.
2) Which is the stronger base? There's two to choose from – one on each side.
НРо
Н,о (снсон Nн,
64
7.0 92
9.3
10.0
12.3
15.7
19
34
3) Which side of the equilibrium is favored?
(CH)CОН + он-
(CH:)»CO + H;O
HF + CH:CO;Na
NaF + CH:CO;H
H2S + CHsONa
NaSH + CHsOH
NACN + NazHPO4
NasPO. + HCN
H;CO: + NH3 + NaCl
NaHCO: + NH.CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning