5. A 100-mL sample of water containing Ca?* and Mg?* ions is titrated with 15.28 ml of 0.01016 M EDTA in an ammonia buffer at pH 10.00. Another sample of 100 ml is treated with NaOH to precipitate Mg(OH)2, and then titrated at pH 10.00 with 10.43 mL of the same EDTA solution. Calculate the conc in ppm of CaCO3 (100.09) and MGCO3 (84.314) in the sample.
5. A 100-mL sample of water containing Ca?* and Mg?* ions is titrated with 15.28 ml of 0.01016 M EDTA in an ammonia buffer at pH 10.00. Another sample of 100 ml is treated with NaOH to precipitate Mg(OH)2, and then titrated at pH 10.00 with 10.43 mL of the same EDTA solution. Calculate the conc in ppm of CaCO3 (100.09) and MGCO3 (84.314) in the sample.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section4.9: Spectrophotometry
Problem 3.1ACP
Related questions
Question
100%
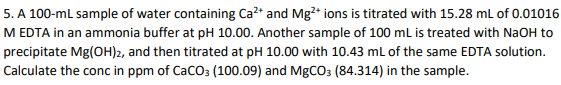
Transcribed Image Text:5. A 100-ml sample of water containing Ca²* and Mg2* ions is titrated with 15.28 mL of 0.01016
M EDTA in an ammonia buffer at pH 10.00. Another sample of 100 mL is treated with NaOH to
precipitate Mg(OH)2, and then titrated at pH 10.00 with 10.43 ml of the same EDTA solution.
Calculate the conc in ppm of CaCO: (100.09) and MgCO3 (84.314) in the sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning