5. A given molecule has a one-dimensional conjugated ¹-electron networ that is distributed to several energy levels following particle in a box energy equation En A) 5 B) 7 C) 10 D) 3 E) 6 = h²n² 8ma² The ratio of the transition energies from the highest occupied (HOMO) to the second-lowest unoccupied (LUMO) relative to the transition to the first-lowest unoccupied (LUMOs) molecular orbital is equal to 16/ What is the total number of the π-electrons in the network?
5. A given molecule has a one-dimensional conjugated ¹-electron networ that is distributed to several energy levels following particle in a box energy equation En A) 5 B) 7 C) 10 D) 3 E) 6 = h²n² 8ma² The ratio of the transition energies from the highest occupied (HOMO) to the second-lowest unoccupied (LUMO) relative to the transition to the first-lowest unoccupied (LUMOs) molecular orbital is equal to 16/ What is the total number of the π-electrons in the network?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter12: The Solid State
Section: Chapter Questions
Problem 19PS: Considering only the molecular orbitals formed by combinations of the 2s atomic orbitals, how many...
Related questions
Question
Solve please.
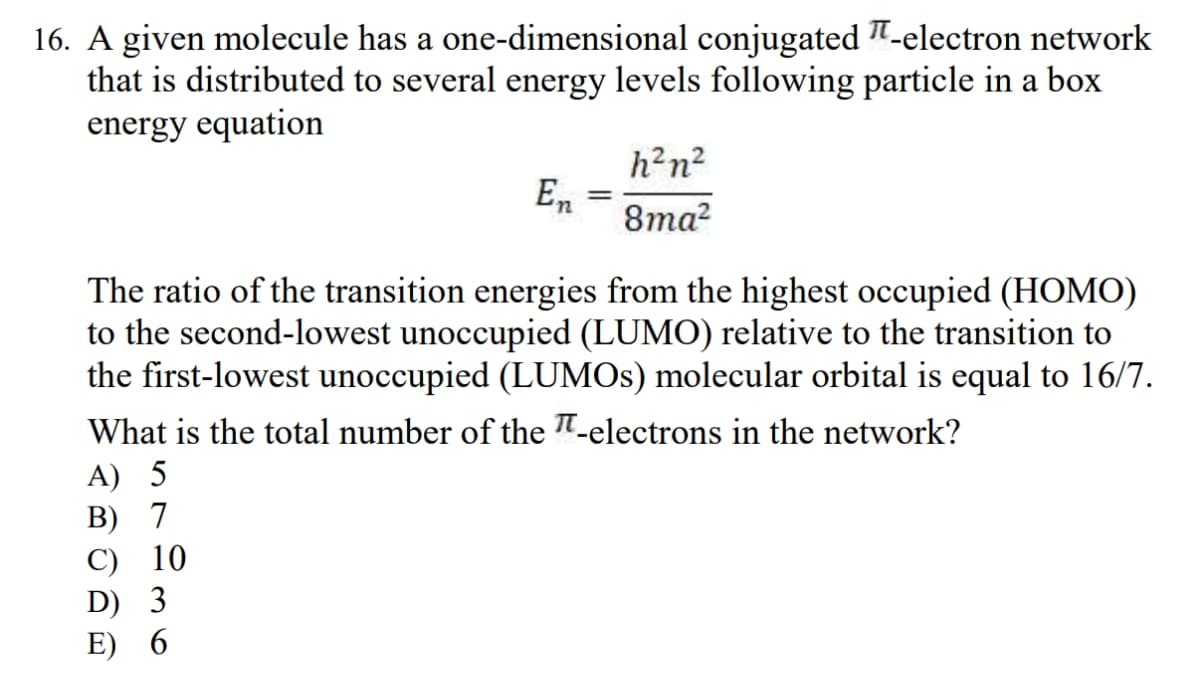
Transcribed Image Text:16. A given molecule has a one-dimensional conjugated -electron network
that is distributed to several energy levels following particle in a box
energy equation
En
C) 10
D) 3
E) 6
=
h²n²
8ma²
The ratio of the transition energies from the highest occupied (HOMO)
to the second-lowest unoccupied (LUMO) relative to the transition to
the first-lowest unoccupied (LUMOs) molecular orbital is equal to 16/7.
What is the total number of the -electrons in the network?
A) 5
B) 7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,