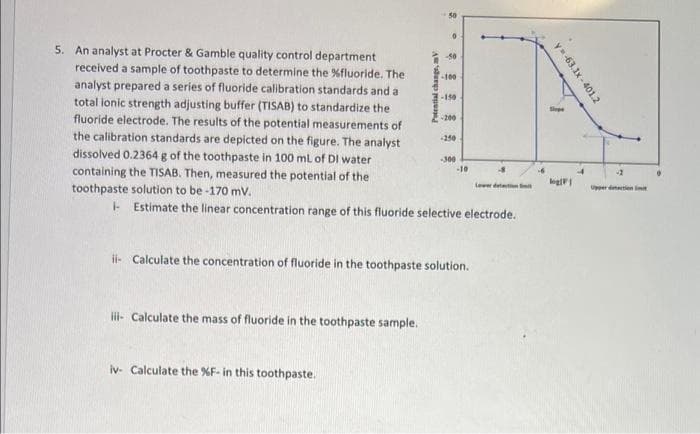
5. An analyst at Procter & Gamble quality control department received a sample of toothpaste to determine the %fluoride. The analyst prepared a series of fluoride calibration standards and a total ionic strength adjusting buffer (TISAB) to standardize the fluoride electrode. The results of the potential measurements of the calibration standards are depicted on the figure. The analyst dissolved 0.2364 g of the toothpaste in 100 ml of DI water containing the TISAB. Then, measured the potential of the toothpaste solution to be-170 mv. -100 -200 10 lewe d - Estimate the linear concentration range of this fluoride selective electrode. Peal chang, V
5. An analyst at Procter & Gamble quality control department received a sample of toothpaste to determine the %fluoride. The analyst prepared a series of fluoride calibration standards and a total ionic strength adjusting buffer (TISAB) to standardize the fluoride electrode. The results of the potential measurements of the calibration standards are depicted on the figure. The analyst dissolved 0.2364 g of the toothpaste in 100 ml of DI water containing the TISAB. Then, measured the potential of the toothpaste solution to be-170 mv. -100 -200 10 lewe d - Estimate the linear concentration range of this fluoride selective electrode. Peal chang, V
Chapter11: Dynamic Electrochemistry
Section: Chapter Questions
Problem 10P
Related questions
Question
Transcribed Image Text:50
5. An analyst at Procter & Gamble quality control department
received a sample of toothpaste to determine the %fluoride. The
analyst prepared a series of fluoride calibration standards and a
total ionic strength adjusting buffer (TISAB) to standardize the
fluoride electrode. The results of the potential measurements of
the calibration standards are depicted on the figure. The analyst
dissolved 0.2364 g of the toothpaste in 100 ml of DI water
containing the TISAB. Then, measured the potential of the
toothpaste solution to be -170 mv.
-100
-150
-200
-250
300
10
Lewer deteton
Uer detten
- Estimate the linear concentration range of this fluoride selective electrode.
i- Calculate the concentration of fluoride in the toothpaste solution.
li- Calculate the mass of fluoride in the toothpaste sample.
iv- Calculate the %F- in this toothpaste.
y-63.1x - 401.2
Potential change, mV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole



Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning