5. Answer the following questions a-d: Type all questions and answers. a. i) Look at your results for Table II. Which one of the three buffers was the best buffer overall? ii) Look at the number of drops. Which solution preparation was the best buffer against HCI? iii) Which was the best buffer against NaOH? b. Looking at the method of preparation and molarities in the prepared solutions; Explain why you think the particular preparation chosen in answer (a) above buffered better against HCl or NaOH. c. Using your text book or internet sources, summarize the use of another chemical buffer in our body, environment, or industrial usage THAT WAS NOT MENTIONED IN THIS EXPERIMENT. Include your reference. Just saying "so and so is a buffer" does not answer this question. Your answer should include appropriate reactions as well. d. Was water a good buffer? Explain your answer. Be careful to not just restate the facts.
5. Answer the following questions a-d: Type all questions and answers. a. i) Look at your results for Table II. Which one of the three buffers was the best buffer overall? ii) Look at the number of drops. Which solution preparation was the best buffer against HCI? iii) Which was the best buffer against NaOH? b. Looking at the method of preparation and molarities in the prepared solutions; Explain why you think the particular preparation chosen in answer (a) above buffered better against HCl or NaOH. c. Using your text book or internet sources, summarize the use of another chemical buffer in our body, environment, or industrial usage THAT WAS NOT MENTIONED IN THIS EXPERIMENT. Include your reference. Just saying "so and so is a buffer" does not answer this question. Your answer should include appropriate reactions as well. d. Was water a good buffer? Explain your answer. Be careful to not just restate the facts.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.7QE
Related questions
Question
Help me with question A-D

Transcribed Image Text:5. Answer the following questions a-d: Type all questions and answers.
a. i) Look at your results for Table II. Which one of the three buffers was
the best buffer overall?
ii) Look at the number of drops. Which solution preparation was the best
buffer against HCI?
iii) Which was the best buffer against NaOH?
b. Looking at the method of preparation and molarities in the prepared
solutions; Explain why you think the particular preparation chosen in
answer (a) above buffered better against HCl or NaOH.
c. Using your text book or internet sources, summarize the use of another
chemical buffer in our body, environment, or industrial usage THAT
WAS NOT MENTIONED IN THIS EXPERIMENT. Include your
reference. Just saying "so and so is a buffer" does not answer this
question. Your answer should include appropriate reactions as well.
d. Was water a good buffer? Explain your answer. Be careful to not just
restate the facts.
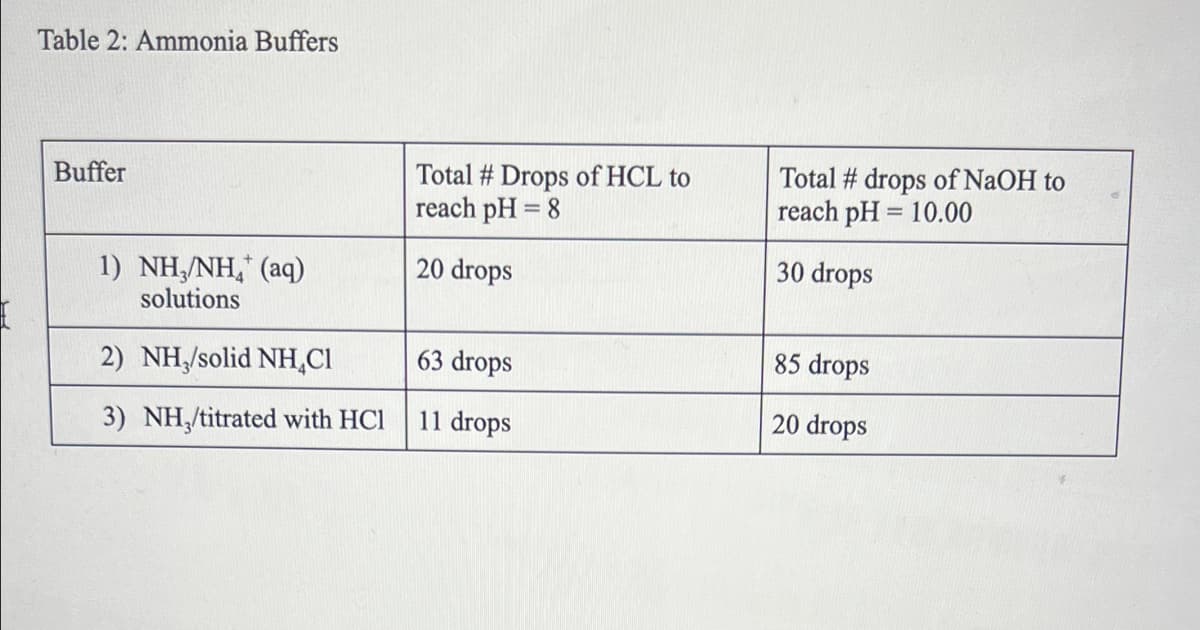
Transcribed Image Text:Table 2: Ammonia Buffers
Total # Drops of HCL to
reach pH = 8
Total # drops of NaOH to
reach pH = 10.00
Buffer
20 drops
30 drops
1) NH,/NH, (aq)
solutions
2) NH/solid NH,CI
63 drops
85 drops
3) NH,/titrated with HCl
11 drops
20 drops
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning
