In part one if you allow your titration to go beyond a faint pink endpoint and it ends up dark pink, what affect will that have on your calculated molarity of NaOH? Would you calculate a molarity that is higher than/lower than/the same as the actual molarity? Explain your reasoning. | I What affect, in any, would this error have on the molarity of acid in part two? Would you calculate a molarity that is higher than/lower than/the same as the actual molarity? Explain your reasoning. Can you confidently determine if your molarities are accurate? Explain why or why not. Can you confidently determine if your molarities are precise? Explain why or why not.
In part one if you allow your titration to go beyond a faint pink endpoint and it ends up dark pink, what affect will that have on your calculated molarity of NaOH? Would you calculate a molarity that is higher than/lower than/the same as the actual molarity? Explain your reasoning. | I What affect, in any, would this error have on the molarity of acid in part two? Would you calculate a molarity that is higher than/lower than/the same as the actual molarity? Explain your reasoning. Can you confidently determine if your molarities are accurate? Explain why or why not. Can you confidently determine if your molarities are precise? Explain why or why not.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.26QAP
Related questions
Question
I need answer for question 1 2 3 and 4 based on the finished titration data sheet
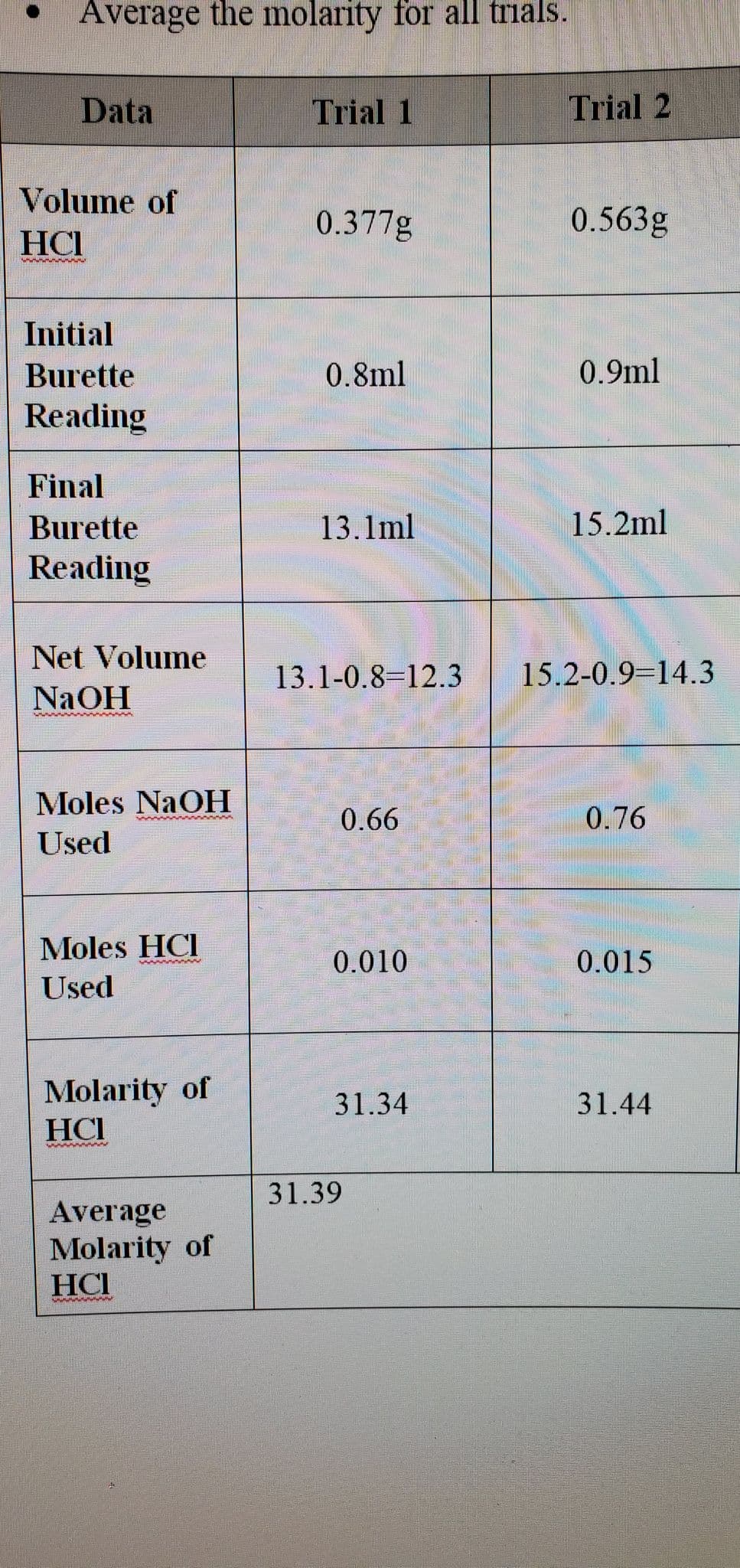
Transcribed Image Text:Average the molarity for all trials.
Data
Volume of
HCI
MA
Initial
Burette
Reading
Final
Burette
Reading
Net Volume
NaOH
Moles NaOH
Used
Moles HCI
Used
www.
Molarity of
HCI
www
Average
Molarity of
HCI
www.
Trial 1
0.377g
0.8ml
13.1ml
13.1-0.8-12.3
0.66
0.010
31.34
31.39
Trial 2
0.563g
0.9ml
15.2ml
15.2-0.9 14.3
0.76
0.015
31.44
![268]
63
E
Euro
151
1. In part one if you allow your titration to go beyond a faint pink endpoint and it ends up dark pink, what
affect will that have on your calculated molarity of NaOH? Would you calculate a molarity that is higher
than/lower than/the same as the actual molarity? Explain your reasoning. |
I
2. What affect, in any, would this error have on the molarity of acid in part two? Would you calculate a
molarity that is higher than/lower than/the same as the actual molarity? Explain your reasoning.
3. Can you confidently determine if your molarities are accurate? Explain why or why not.
4. Can you confidently determine if your molarities are precise? Explain why or why not.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fcbc9cffe-f157-4023-ae1e-1819ef0454cd%2F15441c54-e831-4301-bdbf-de10eee02bec%2Fzn6cvp_processed.jpeg&w=3840&q=75)
Transcribed Image Text:268]
63
E
Euro
151
1. In part one if you allow your titration to go beyond a faint pink endpoint and it ends up dark pink, what
affect will that have on your calculated molarity of NaOH? Would you calculate a molarity that is higher
than/lower than/the same as the actual molarity? Explain your reasoning. |
I
2. What affect, in any, would this error have on the molarity of acid in part two? Would you calculate a
molarity that is higher than/lower than/the same as the actual molarity? Explain your reasoning.
3. Can you confidently determine if your molarities are accurate? Explain why or why not.
4. Can you confidently determine if your molarities are precise? Explain why or why not.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning