5. Assign the appropriate formal charge to the indicated atoms. Here, lone pairs are shown for learning and clarity. In other words, consider the structure as drawn. In the future, only the charge will be drawn. Н NH2 Н .N'
5. Assign the appropriate formal charge to the indicated atoms. Here, lone pairs are shown for learning and clarity. In other words, consider the structure as drawn. In the future, only the charge will be drawn. Н NH2 Н .N'
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 45EQ
Related questions
Question
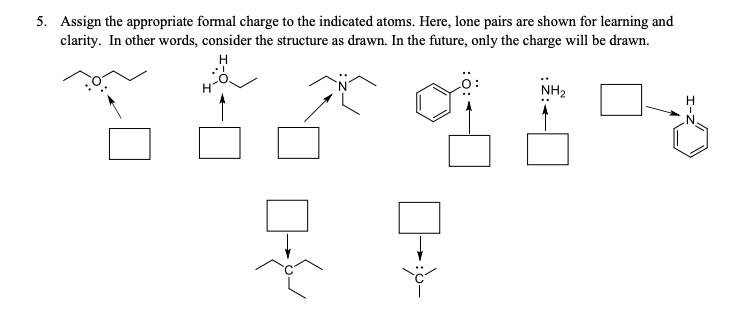
Transcribed Image Text:5. Assign the appropriate formal charge to the indicated atoms. Here, lone pairs are shown for learning and
clarity. In other words, consider the structure as drawn. In the future, only the charge will be drawn.
Н
NH2
Н
.N'
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning