5. Examine the following table and identify which of the substances A to C are ionic, which are polar covalent, and which are non-polar covalent. Give a reason for your choice in each case. Substance Melting Point (°C) Solubility in water A -178 insolubie B -101 soluble C 730 soluble
5. Examine the following table and identify which of the substances A to C are ionic, which are polar covalent, and which are non-polar covalent. Give a reason for your choice in each case. Substance Melting Point (°C) Solubility in water A -178 insolubie B -101 soluble C 730 soluble
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 99AP: A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and...
Related questions
Question
Question number 5 please
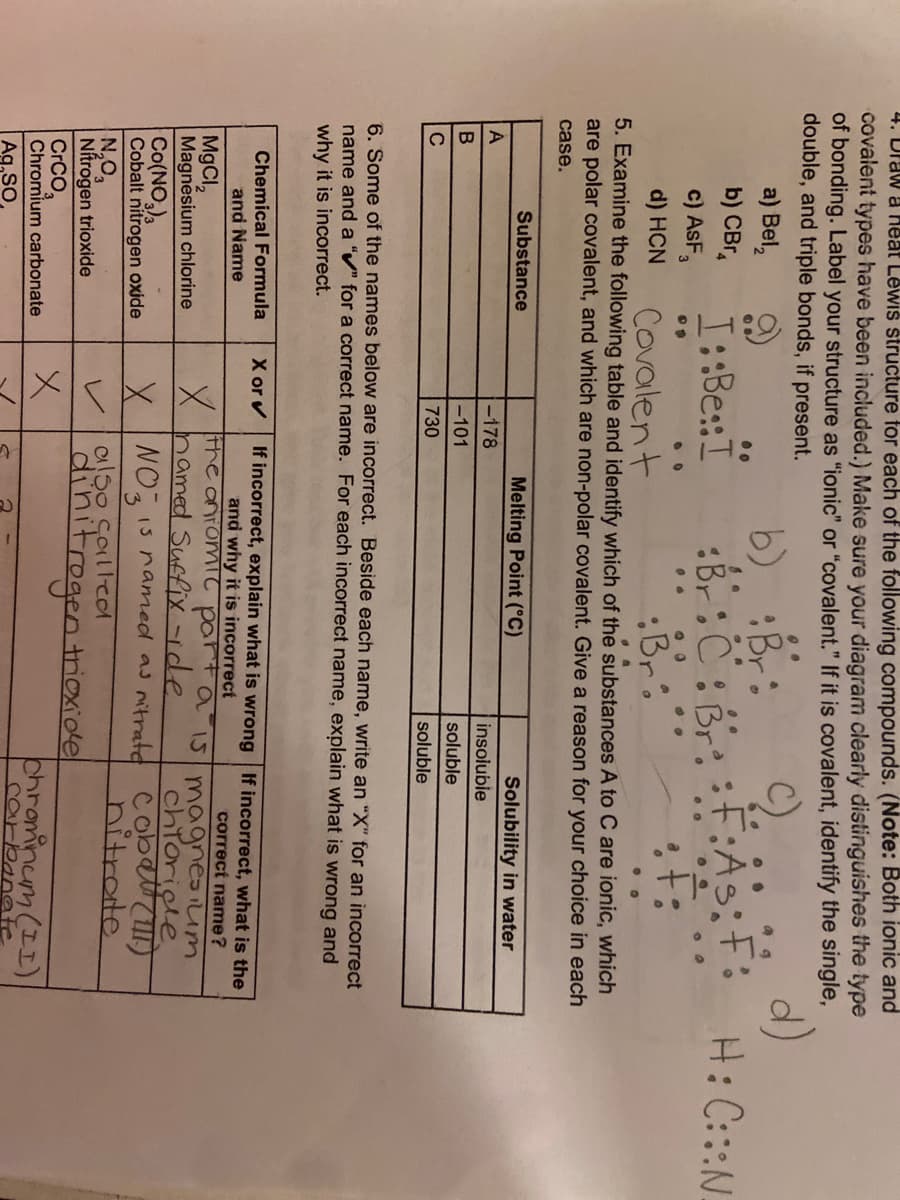
Transcribed Image Text:4. Draw a Heat Lewis structure for each of the following compounds. (Note: Both ionic and
covalent types have been included.) Make sure your diagram clearly distinguishes the type
of bonding. Label your structure as "ionic" or "covalent." If it is covalent, identify the single,
double, and triple bonds, if present.
a) Bel,
b)
Br
:F:As;F:
H: C:::N.
%3B
b) CBr,
c) AsF,
I::Be::I
:Br:C:Br:
..
d) HCN
Covalent
:Br
5. Examine the following table and identify which of the substances A to C are ionic, which
are polar covalent, and which are non-polar covalent. Give a reason for your choice in each
case.
Substance
Melting Point (°C)
Solubility in water
A
-178
insoluble
-101
soluble
C
730
soluble
6. Some of the names below are incorrect. Beside each name, write an "X" for an incorrect
name and a “v" for a correct name. For each incorrect name, explain what is wrong and
why it is incorrect.
X or v
If incorrect, explain what is wrong If incorrect, what is the
Chemical Formula
and Name
and why it is incorrect
MgCI,
Magnesium chlorine
correct name?
the antomit parta iI5 magnesIum
hamed Suffix -ide
chioriele
Co(NO,),
Cobalt nitrogen oxide
X NO IS named as mitratd cobet()
hitrate
Soilled
N,O,
Nítrogen trioxide
CrCO,
Chromium carbonate
nitrogen trioxide
レ
chrominum (II)
carbanate
Ag,SO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax