5. Gas Power Cycle in a Closed System with External Heat Exchange Air in a closed system undergoes the following reversible three-step cycle: 1-2 Isothermal compression of air at T1 = 27°C . The initial pressure is p1 = 2bar 2-3 Isochoric heating to T3 = 1200K . 3-1 Adiabatic reversible expansion to state 1. kJ Consider air as ideal gas with variable specific heats, and gas constant R = 0.287 kgK As you solve the problem, populate the table with the data you need. Use free columns as you like.
5. Gas Power Cycle in a Closed System with External Heat Exchange Air in a closed system undergoes the following reversible three-step cycle: 1-2 Isothermal compression of air at T1 = 27°C . The initial pressure is p1 = 2bar 2-3 Isochoric heating to T3 = 1200K . 3-1 Adiabatic reversible expansion to state 1. kJ Consider air as ideal gas with variable specific heats, and gas constant R = 0.287 kgK As you solve the problem, populate the table with the data you need. Use free columns as you like.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Hi there,
I only need answers for d) and e). Data is filled out in second image. Thanks
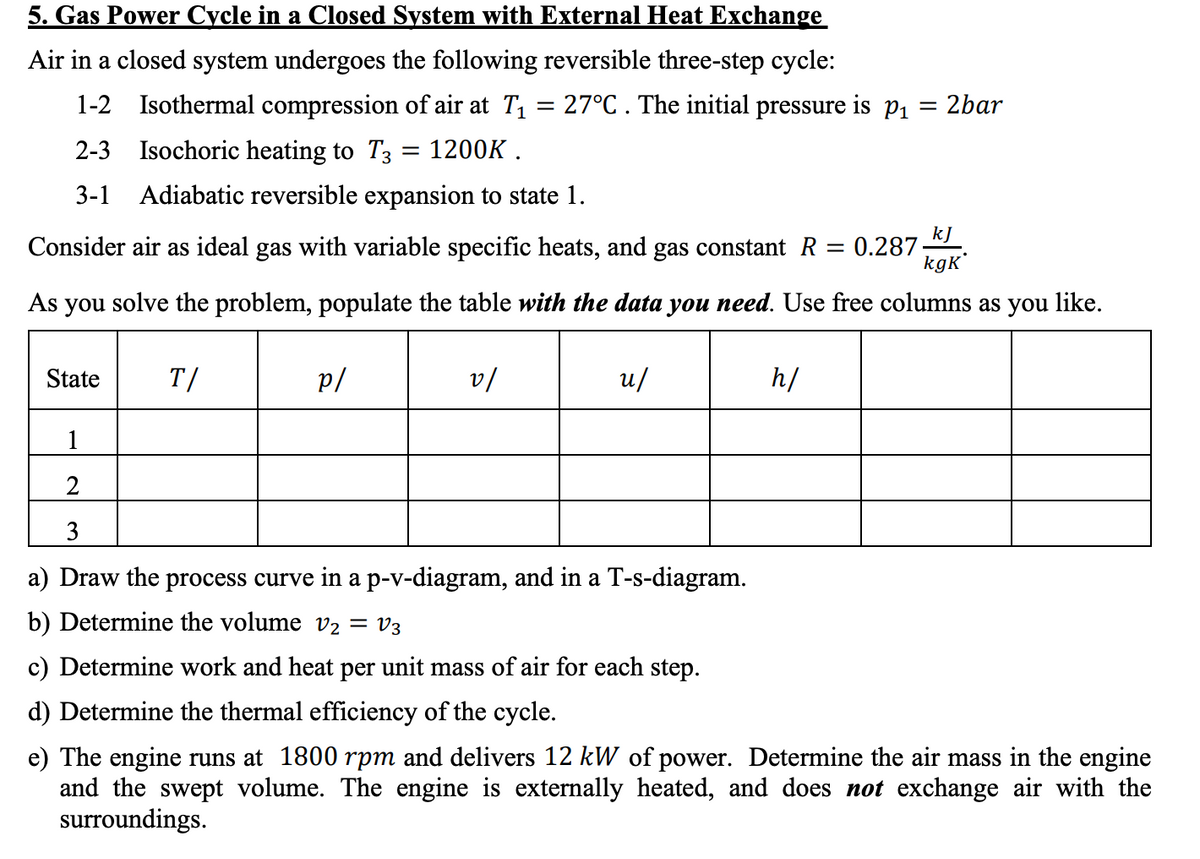
Transcribed Image Text:5. Gas Power Cycle in a Closed System with External Heat Exchange
Air in a closed system undergoes the following reversible three-step cycle:
1-2 Isothermal compression of air at T1 = 27°C. The initial pressure is p1 = 2bar
2-3 Isochoric heating to T3 = 1200K .
%3D
3-1
Adiabatic reversible expansion to state 1.
Consider air as ideal gas with variable specific heats, and gas constant R
kJ
0.287
kgK'
As you solve the problem, populate the table with the data you need. Use free columns as you like.
State
T/
p/
v/
u/
h/
1
2
3
a) Draw the process curve in a p-v-diagram, and in a T-s-diagram.
b) Determine the volume V2 = V3
c) Determine work and heat per unit mass of air for each step.
d) Determine the thermal efficiency of the cycle.
e) The engine runs at 1800 rpm and delivers 12 kW of power. Determine the air mass in the engine
and the swept volume. The engine is externally heated, and does not exchange air with the
surroundings.
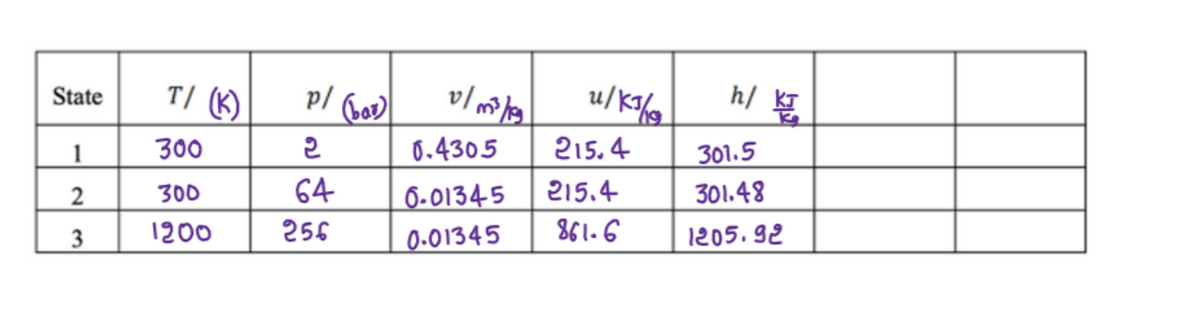
Transcribed Image Text:T/ (K)
p/ Gol
h/
u/K%,
State
1
300
0.4305
215.4
301.5
300
64
6-0134-5
215.4
301.48
3
1200
256
0-01345
861.6
1205. 92
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY