5.) Given the half reactions Cu²*(aq) + 2e → Cu (s) E°red = 0.34 V Pb?* (аq) + 2 е Pb (s) Е ed 3 -0.13 V which is the K. value for the reaction below, when conducted at 25°C? Pb2+ (aq) + Cu (s) → Pb (s) + Cu²+ (aq) I. 7.9 x 10-8 П. 8.9х 107 Ш. 7.9 х 1015 IV. 1.3 x 10-16
5.) Given the half reactions Cu²*(aq) + 2e → Cu (s) E°red = 0.34 V Pb?* (аq) + 2 е Pb (s) Е ed 3 -0.13 V which is the K. value for the reaction below, when conducted at 25°C? Pb2+ (aq) + Cu (s) → Pb (s) + Cu²+ (aq) I. 7.9 x 10-8 П. 8.9х 107 Ш. 7.9 х 1015 IV. 1.3 x 10-16
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 3RQ: Table 17-1 lists common half-reactions along with the standard reduction potential associated with...
Related questions
Question
Please expain it in detail.
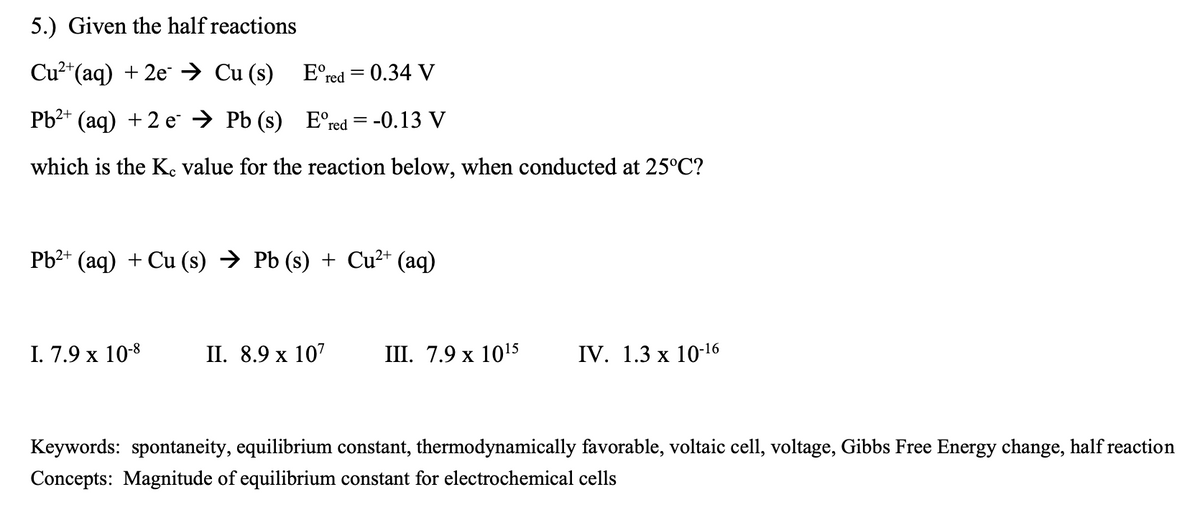
Transcribed Image Text:5.) Given the half reactions
Cu2*(aq) + 2e → Cu (s) E°red = 0.34 V
Рb** (aq) + 2 е > PЬ (s) E'"red 3D -0.13 V
which is the K, value for the reaction below, when conducted at 25°C?
Pb2+ (aq) + Cu (s) → Pb (s) + Cu²* (aq)
I. 7.9 x 10-8
I. 8.9 х 107
I. 7.9 х 1015
IV. 1.3 x 10-16
Keywords: spontaneity, equilibrium constant, thermodynamically favorable, voltaic cell, voltage, Gibbs Free Energy change, half reaction
Concepts: Magnitude of equilibrium constant for electrochemical cells
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning