5. What is the pressure of "wet" oxygen if 0.8 mol of dry oxygen at 101.3 kPa is collected? The atmospheric pressure in the lab that day is 99.4 kPa. The oxygen is to be collected over water at a temperature of 15.0°C. ŁPa HLO 1.7os620019 lsinsq to wsl z'notle0-TyivitoA adt onitslulea gedw ebe
5. What is the pressure of "wet" oxygen if 0.8 mol of dry oxygen at 101.3 kPa is collected? The atmospheric pressure in the lab that day is 99.4 kPa. The oxygen is to be collected over water at a temperature of 15.0°C. ŁPa HLO 1.7os620019 lsinsq to wsl z'notle0-TyivitoA adt onitslulea gedw ebe
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.22E: Earths atmosphere is approximately 80 N2 and 20 O2. If the total atmospheric pressure at sea level...
Related questions
Question
100%
Can you please explain what "wet" oxygen means and how to do the attached question? Thank you!
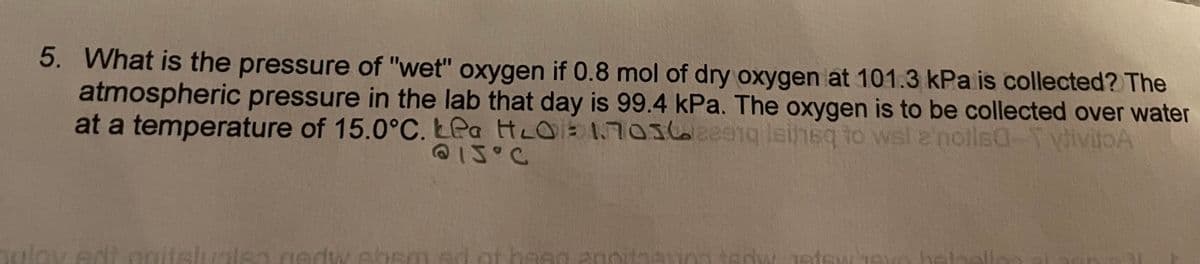
Transcribed Image Text:5. What is the pressure of "wet" oxygen if 0.8 mol of dry oxygen at 101.3 kPa is collected? The
atmospheric pressure in the lab that day is 99.4 kPa. The oxygen is to be collected over water
at a temperature of 15.0°C. LPa HLO1703620e1g leinsq to wsl 2'notlsa-T vivitoA
@1J°C
ov edt onitslualsa.gedw.sbsm ed ot basg a0oitaan0a tedw Jetewjeu
veteel
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning