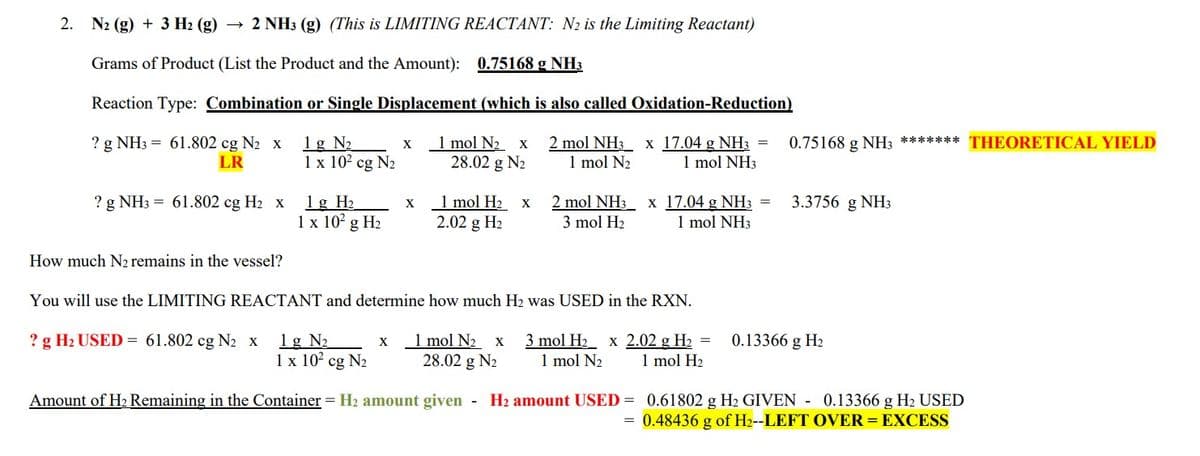
Directions: Write the balanced equation for each of the following situations. SHOW ALL OF YOUR
WORK ON ATTACHED PAGES, OR IT WILL NOT BE ACCEPTED. In addition, list the reaction type.
YOU MUST TELL THE AMOUNTS OF EVERY SUBSTANCE THAT REMAINS IN THE
CONTAINER AT THE END OF THE REACTION. ASSUME THAT ALL REACTIONS
GO TO COMPLETION.
If only STOICHIOMETRY, tell how much of the excess reactant is used!!!!
Reaction Type
a. Combination Reaction
b. Decomposition Reaction
c. Single Displacement / THIS IS ONE TYPE OF
d. Precipitation Reaction
e. Gaseous Reaction
f. Neutralization Reaction
g. Combustion Reaction
5.92 g of sodium oxalate is reacted with 5.92 of calcium chloride
follow the format used in the image provided.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images









