A powder contains FeSO, -7H,O (molar mass=278.01 g/mol), among other components. A 2.595 g sample of the powder was dissolved in HNO, and heated to convert all iron to Fe". The addition of NH, precipitated Fe,0,- xH₂O, which was subsequently ignited to produce 0.530 g Fe,0,. What was the mass of FeSO, 7H₂O in the 2.595 g sample? mass of FeSO, 7H₂O: 1.72
A powder contains FeSO, -7H,O (molar mass=278.01 g/mol), among other components. A 2.595 g sample of the powder was dissolved in HNO, and heated to convert all iron to Fe". The addition of NH, precipitated Fe,0,- xH₂O, which was subsequently ignited to produce 0.530 g Fe,0,. What was the mass of FeSO, 7H₂O in the 2.595 g sample? mass of FeSO, 7H₂O: 1.72
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter4: Reactions In Aqueous Solution
Section: Chapter Questions
Problem 77QAP: A 300.0-g sample of a solid is made up of a uniform mixture of NaNO3, MgCl2, and BaCl2. A 100.0-g...
Related questions
Question
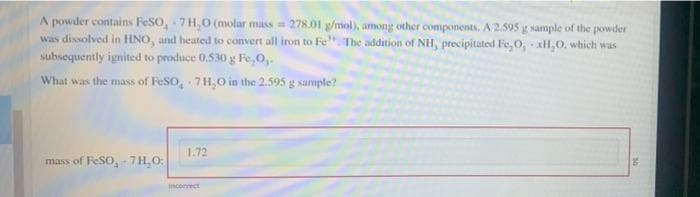
Transcribed Image Text:A powder contains FeSO, -7H,O (molar mass=278.01 g/mol), among other components. A 2.595 g sample of the powder
was dissolved in HNO, and heated to convert all iron to Fe". The addition of NH, precipitated Fe,0,- xH₂O, which was
subsequently ignited to produce 0.530 g Fe,0,.
What was the mass of FeSO, 7H₂O in the 2.595 g sample?
mass of FeSO, 7H₂O:
1.72
incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning