53. Which compound has the higher boiling point CH3CH2CH2OCH2CH3 CH3CH2CH2COOH fropanoie ocid CH3CH2COOH CH3C a CHECHCH2CH2OH 54. Which is more soluble in octane? CH2CH2COOH A 55. Which has lower boiling point? A butyrate butyric acid 56. Which is less soluble in water?
53. Which compound has the higher boiling point CH3CH2CH2OCH2CH3 CH3CH2CH2COOH fropanoie ocid CH3CH2COOH CH3C a CHECHCH2CH2OH 54. Which is more soluble in octane? CH2CH2COOH A 55. Which has lower boiling point? A butyrate butyric acid 56. Which is less soluble in water?
Chapter17: Alcohols And Phenols
Section17.2: Properties Of Alcohols And Phenols
Problem 3P: The following data for isomeric four-carbon alcohols show that there is a decrease in boiling point...
Related questions
Question
Please give the correct answer.
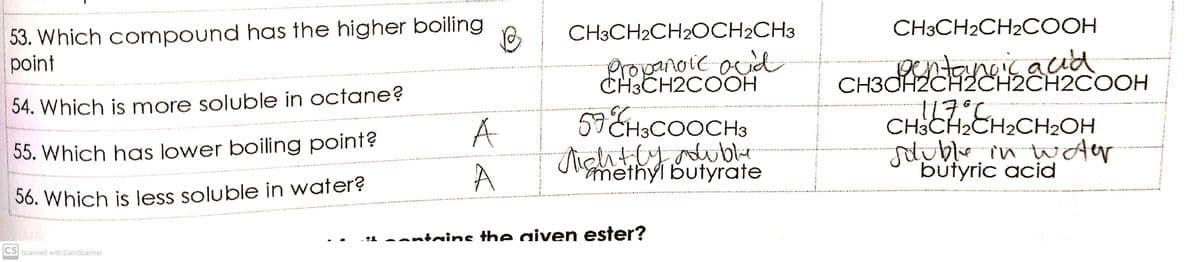
Transcribed Image Text:53. Which compound has the higher boiling
point
CH3CH2CH2OCH2CH3
CH3CH2CH2COOH
Propanoic ocid
CH3CH2COOH
CH3CP aud
2CH2CH2CH2COOH
54. Which is more soluble in octane?
A
57 CHsCOOCH3
CH3CH2CH2CH2OH
55. Which has lower boiling point?
A
methyl butyrate
butyric acid
36. Which is less soluble in water?
-1 -antrine the aiven ester?
CS Scanned with CamScanner
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning