IV. Which member of each pair has the higher boiling point? Explain briefly your answers. 1. CH3CH2CH2CH2CH3 or CH3CH2CH2CH2CH2CH3 2. H20 or HBr 3. HO-CH2-CH2OH or CH3CH2OH
IV. Which member of each pair has the higher boiling point? Explain briefly your answers. 1. CH3CH2CH2CH2CH3 or CH3CH2CH2CH2CH2CH3 2. H20 or HBr 3. HO-CH2-CH2OH or CH3CH2OH
Organic And Biological Chemistry
7th Edition
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:STOKER, H. Stephen (howard Stephen)
Chapter6: Amines And Amides
Section: Chapter Questions
Problem 6.38EP
Related questions
Question
please answer number 2
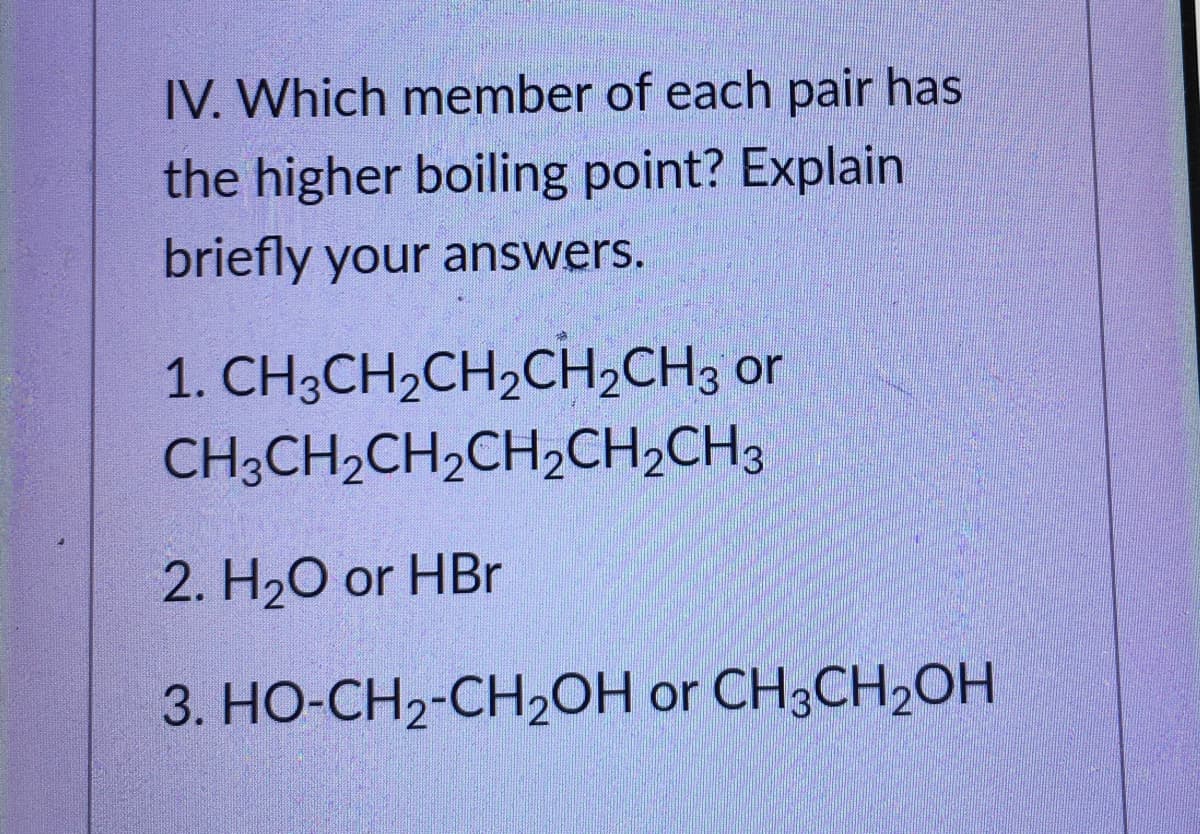
Transcribed Image Text:IV. Which member of each pair has
the higher boiling point? Explain
briefly your answers.
1. CH3CH2CH,CH,CH3 or
CH;CH2CH2CH2CH,CH3
2. H20 or HBr
3. HO-CH2-CH20H or CH3CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:
9781305081079
Author:
STOKER, H. Stephen (howard Stephen)
Publisher:
Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning