54. The correct name for the compound given be low is which of the follow ing? -CCH,CH,CH3 1-pheny-1-butanone b. pheny lbutanal a. c. 4-pheny-4-butanone d. butanonebenzene 55. The functional group of a molecule does not affect a compound's a. solubility b. melt ing point с. reaction with other molecules d. carbon chain length 50. The met ing point ofa compound is not influenced by which of the following? a. polarity b. electronegativity of its atoms Van der Waals forçes d. hydrogen bonds e. isotope of carbon с.
54. The correct name for the compound given be low is which of the follow ing? -CCH,CH,CH3 1-pheny-1-butanone b. pheny lbutanal a. c. 4-pheny-4-butanone d. butanonebenzene 55. The functional group of a molecule does not affect a compound's a. solubility b. melt ing point с. reaction with other molecules d. carbon chain length 50. The met ing point ofa compound is not influenced by which of the following? a. polarity b. electronegativity of its atoms Van der Waals forçes d. hydrogen bonds e. isotope of carbon с.
Chapter4: Organic Compounds: Cycloalkanes And Their Stereochemistry
Section4.3: Stability Of Cycloalkanes: Ring Strain
Problem 8P: Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are...
Related questions
Question
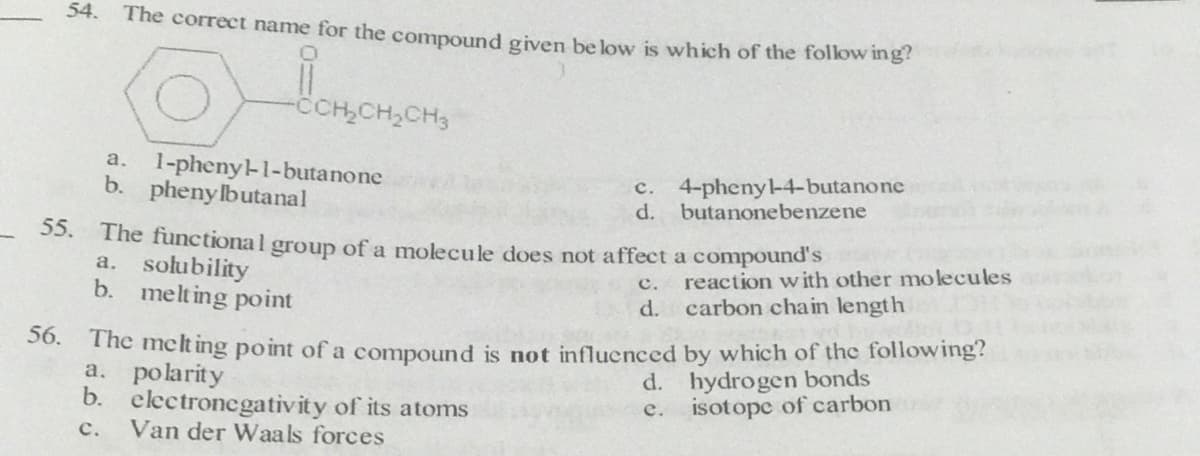
Transcribed Image Text:54.
The correct name for the compound given be low is which of the follow ing?
-CCH,CH,CH3
1-pheny-1-butanone
b. pheny lbutanal
a.
c. 4-pheny-4-butanone
d. butanonebenzene
55. The functional group of a molecule does not affect a compound's
a. solubility
b. melting point
c. reaction with other molecules
d. carbon chain length
50. The meclt ing point of a compound is not influenced by which of the following?
a. polarity
b. electronegativity of its atoms
Van der Waals forces
d. hydrogen bonds
isotope of carbon
e.
с.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co