59. An adult eats food whose nutritional energy totals approxi- mately 2.2 x 10 Cal per day. The adult burns 2.0 × 10° Cal per day. How much excess nutritional energy, in kilojoules, does the adult consume per day? If 1 lb of fat is stored by the body for each 14.6 × 10° kJ of excess nutritional energy consumed, how long will it take this person to gain 1 lb?
59. An adult eats food whose nutritional energy totals approxi- mately 2.2 x 10 Cal per day. The adult burns 2.0 × 10° Cal per day. How much excess nutritional energy, in kilojoules, does the adult consume per day? If 1 lb of fat is stored by the body for each 14.6 × 10° kJ of excess nutritional energy consumed, how long will it take this person to gain 1 lb?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.70QP: An iron skillet weighing 1.63 kg is heated on a stove to 178C. Suppose the skillet is cooled to room...
Related questions
Question
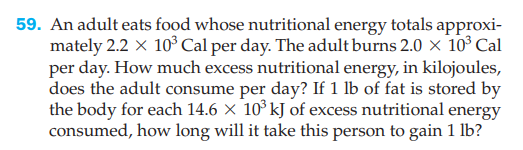
Transcribed Image Text:59. An adult eats food whose nutritional energy totals approxi-
mately 2.2 x 10 Cal per day. The adult burns 2.0 × 10° Cal
per day. How much excess nutritional energy, in kilojoules,
does the adult consume per day? If 1 lb of fat is stored by
the body for each 14.6 × 10° kJ of excess nutritional energy
consumed, how long will it take this person to gain 1 lb?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning