6. Breaking peptide bonds is hard. a) Draw steps for (i) addition of water and (ii) elimination of the amine from the CO-NH bond of R-CO-NH-R' ("the reactant"). Include any intermediate steps like proton transfer. b) Draw a resonance structure for the reactant. c) What is the hybridization of the reactant's indicated (i) C, (ii) N, and (iii) O?
6. Breaking peptide bonds is hard. a) Draw steps for (i) addition of water and (ii) elimination of the amine from the CO-NH bond of R-CO-NH-R' ("the reactant"). Include any intermediate steps like proton transfer. b) Draw a resonance structure for the reactant. c) What is the hybridization of the reactant's indicated (i) C, (ii) N, and (iii) O?
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter18: Aromaticity
Section: Chapter Questions
Problem 18E
Related questions
Question
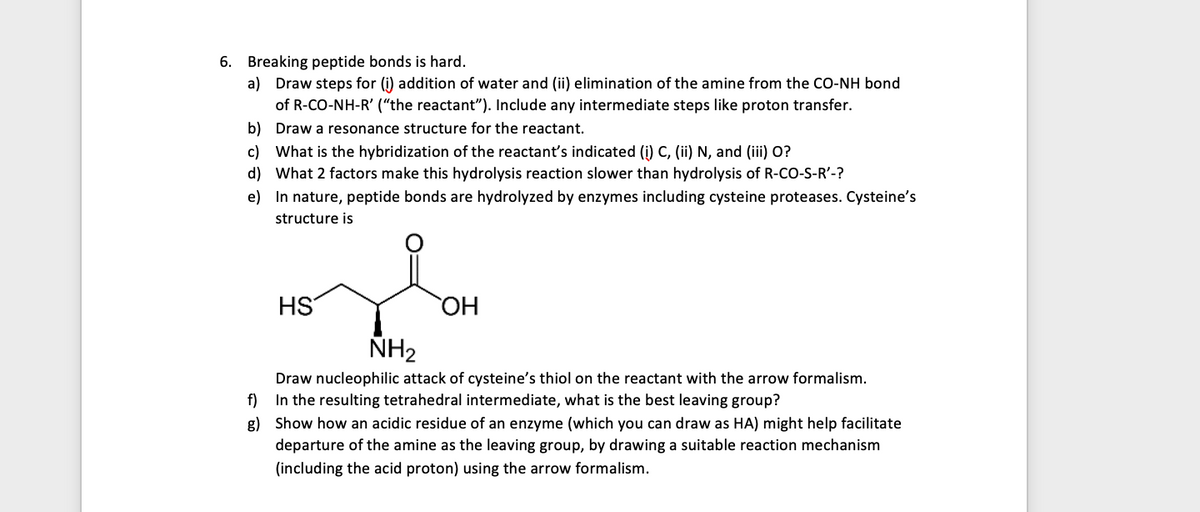
Transcribed Image Text:6. Breaking peptide bonds is hard.
a) Draw steps for (i) addition of water and (ii) elimination of the amine from the CO-NH bond
of R-CO-NH-R' ("the reactant"). Include any intermediate steps like proton transfer.
b) Draw a resonance structure for the reactant.
c) What is the hybridization of the reactant's indicated (i) C, (ii) N, and (iii) O?
d) What 2 factors make this hydrolysis reaction slower than hydrolysis of R-co-S-R'-?
e) In nature, peptide bonds are hydrolyzed by enzymes including cysteine proteases. Cysteine's
structure is
HS
HO
ÑH2
Draw nucleophilic attack of cysteine's thiol on the reactant with the arrow formalism.
f) In the resulting tetrahedral intermediate, what is the best leaving group?
g) Show how an acidic residue of an enzyme (which you can draw as HA) might help facilitate
departure of the amine as the leaving group, by drawing a suitable reaction mechanism
(including the acid proton) using the arrow formalism.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning