6. Calculate Kc for 2N,O4e) + 30zle) → 2N,0 (2 K=? using. (E) Reaction 1 2N218) + Ozle) * 2N,0 (e) K, = 1.2 x 10 35 Reaction 2 - 2NO2 (e) K2 = 4.6 x 103 Reaction 3 ½ Nze) + O2le) → NO2(e) K3 = 4.1 x 10
Q: 2) Provide the reagent(s) and the reaction mechanism of the following reaction. (* is the radio…
A: Detail mechanistic pathway is given below to find out the correct reagents for following conversion
Q: In the following net ionic equation, identify the Bronsted-Lowry acid: NO2−(aq) + HS−(aq) HNO2(aq)…
A:
Q: At 1000°C, the reaction NO2(3) + SO23) mia NO(g) + SO318) has Ke- 4.57. Suppose 0.160 mol of NO2 and…
A: Given data Reaction: NO2 (g)+SO2 (g)⇔NO (g)+SO3 (g) Initial mole of gases are NO2=0.160…
Q: High performance liquid chromatography can separate eluates even if they are not present in the UV…
A: Here, we need to compare HPLC and UV detection techniques.
Q: Which of the following reagent is used for hydroboration? a Phosphoric acid b Tetrahydrofuran…
A: ★ Answer : Correct option is (b) Tetrahydrofuran. ★ Hydroboration reaction is a two-step hydration…
Q: n Mass Spectrometry, what high energy particle is responsible is used to bombard molecule so that it…
A:
Q: The equilibrium constant, Kp, for the following reaction is 0.497 at 500K. PCI5(9) PCI3(g) + Cl2(g)…
A: Answer: Given data at the equilibrium of thermal decomposition of PCl5…
Q: Question 4 Which significant information given below CANNOT be derived from mass spectra? molecular…
A: Mass spectra used for determine the exact molecular weight of the sample , unknown compound
Q: Write the balanced reaction between AgNO3 and Bromide
A:
Q: How many gallons of octane, C3H18. having a density of 0.703 g/cm?, would have to be burned to…
A:
Q: Draw the major organic product of the reaction conditions. Show stereochemistry in your structure.…
A: Major organic product of the given reaction = ?
Q: In a reverse phase chromatography setup, which of the following compounds will have the HIGHEST…
A:
Q: Match the given parts with the correct function integrator A. holds as the mobile phase detector B.…
A: In the given question, functions of several units of a standard hplc system are clearly discussed.
Q: A photon has an energy of 2.35 x 10–19 kJ. Find the wave length of the radiation in µm.
A: Energy of photon = 2.35 × 10-19 KJ Wavelength of radiation (in μm) = ?
Q: What reagents are needed in reaction 3? ... Br Br Reaction 1 Reaction 2 Reaction 3 CH3B AIBr3 (A)…
A:
Q: At 100.0 °C, K. = 0.135 for the reaction 3H2(3) + Ngls) = 2NH3g) Ina reaction mixture at equilibrium…
A: Answer: In these questions, to find out the partial pressure of gases following ideal gas equation…
Q: Gas chromatography cannot analyze mixtures due to the sensitivity of the instrument. O True O False
A: The statement, "Gas chromatography cannot analyze mixtures due to the sensitivity of the instrument"…
Q: If a non-polar mobile phase is used in a non-polar stationary phase, the polar components in a…
A: In the given question, the nature of chromatograpy is discussed based on the polarity concept of…
Q: The blue color in fireworks is often achieved by heating copper (1) chloride, CuCl, to about 1200°…
A:
Q: Solve the word problem and show your detailed solution. The final answer should have a unit and the…
A: Mass of aluminium block = 210 g Initial temperature of aluminium block = 55oC Mass of ice = 360 g…
Q: sulated, rigid tank contains 1.9 kg of helium at 30 °C and 580 kPa. A paddle wheel, with a power…
A:
Q: Why is it that one mole of carbon with one mole of oxygen results to one mole carbon dioxide. with…
A: A substance that contains as many particles as there are in exactly 12 g of the 12C isotope is…
Q: What is the H3O* | and the pH of a benzoic acid-benzoate buffer that consists of 0.13 M C6H5COOH and…
A: Given: Concentration of C6H5COOH = 0.13 M Concentration of C6H5COONa = 0.30 M Ka of C6H5COOH =…
Q: Which of the following substituent is an ortho/para-directing deactivator? a amino group b…
A: Which one of the following is correct
Q: 1. Write down the chemical equation for the synthesis of isopentyl acetate. 2. Ethyl methanoate, an…
A: We have find out the chemical equation.
Q: What is the purpose of filling the calorimeter with oxygen? Explain. (maximum of 2 sentences).
A: Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: Which of the following is directly related to reaction rate? A. Concentration B. Pressure C. Time D.…
A: Which of the following is directly related to reaction rate ? A. Concentration B. Pressure C.…
Q: at is the purpose oI vashing bonate? Explain, using equatio
A:
Q: Rank the solubilities (from least to most soluble) of BaCO3, CdS, NiCO3, AgCN, and SnS and explain…
A: Ksp (solubility product constant) ====> gives us the information about how much our compound can…
Q: The rate constant for the reaction is 0.420 M.s at 200 C. A- products If the initial concentration…
A:
Q: What product is expected after reaction 2? ... Reaction 1 Reaction 2 HO A Secondary alcohol B)…
A:
Q: The age of wine can be determined by measuring the trace amount of radioactive tritium, 3H, present…
A: N(t)=N0 exp[-k t]N(t) is the number of radioactive nuclei remaining at time t, N0 is the initial…
Q: What is the solubility product expression for Sn(l03)2? Ksp = [Sn²*][1O3 12 Ksp = [Sn**][210,2 12…
A:
Q: Calculate for the average %moisture content of the granola bar.
A: We know, %moisture content (Wet basis) Mw =(wet weight-dry weight)wet weight×100 Replicate-1: %Mw…
Q: Many metals will corrode or tarnish i.e. they react with oxygen or moisture in air to form a layer…
A:
Q: A buffer that contains 0.578 M base, B, and 0.299 M of its conjugate acid, BH*, has a pH of 9.09.…
A:
Q: integrator A. measures absorbance data detector B. processes data into the display unit injection…
A:
Q: One possible mechanism for the decomposition of ethane, C2H6, into ethylene, C2H4, and hydrogen,…
A: Given- C2H6 -> C2H4 + H2 ->Step in which free radical generated that is initiation step…
Q: Given is the mass spectra of n-octane whre peaks are labeled accordingly. Give the letter that…
A: We have to predict the peak correspond to the given questions.
Q: What happens at the anode in the voltaic cell? Cathode?
A: A voltaic cell is an electrochemical cell that uses a chemical reaction to generate electrical…
Q: Alkenes and alkynes behave like . and thus attract, A nucleophiles; electrophiles B nucleophiles;…
A:
Q: Given is the mass spectra of n-octane whre peaks are labeled accordingly. Give the letter that…
A: Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: Describe atomic structure; name and describe the parts of atoms, and their arrangement/location and…
A: The atom is the smallest unit of matter that may be not divided into smallest particle without…
Q: Which solution has the greatest buffer capacity? Which solution can react with the largest amount…
A: Buffer solution: The solution that resists the change in pH is known as a buffer solution. The…
Q: When 25.0 mL of a 8.39x10 M potassium phosphate solution is combined with 12.0 ml. of a 4.85X10M…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Teeम ठेएवांए व ताटवला ल! &माकणा एल! &XPकाका The pH Scate is व लया न, &plqा०n ; Re loltue Soluilng जी…
A: The pH (power of hydrogen ) is the the measurement of acidity or basicity of a substance . pH = -…
Q: Reaction 1 Reaction 2 HO A Hydration B Hydroboration C Halohydrin formation D Tautomerization
A: Which one of the following is correct
Q: 1. Methanol (CH3OH) is often used as a fuel in high-performance engines in race cars. Using the data…
A: CH3OH(l) + (3/2)O2 (g) CO2 (g)+ 2H2O(l) Standard enthalpy of Combustion = {HF(CO2(g)) +2 x…
Q: For the theoretical reaction: A+ 2 B → D+F a plausible 2-step reaction mechanism was determined to…
A: The mechanism of the reaction : 1. A + B <---> C + D 2. B + C ---> D (slow) Based on…
Q: Chemistry Calculate [Cu2+]eq using Nernst Equation E cell: 0.48 Temperature: 25.0 C n=2
A: EoCu²+/Cu : 0.337 V Given, Ecell : 0.48 V Temperature : 25 oC = 298K n = 2
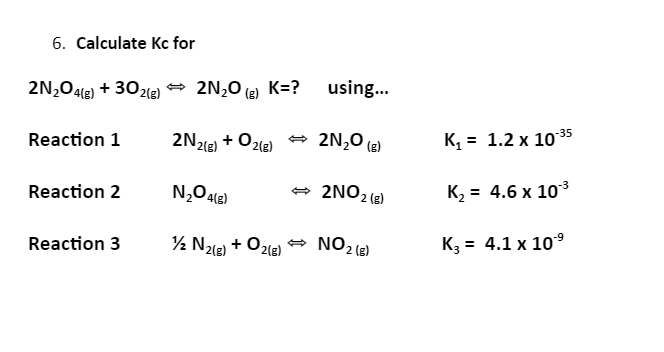
Step by step
Solved in 2 steps

- Write the expression for Kc for the following reactions. Ineach case indicate whether the reaction is homogeneousor heterogeneous.(a) 3 NO1g2 ∆ N2O1g2 + NO21g2(b) CH41g2 + 2 H2S1g2 ∆ CS21g2 + 4 H21g2(c) Ni1CO241g2 ∆ Ni1s2 + 4 CO1g2(d) HF1aq2 ∆ H+1aq2 + F-1aq2(e) 2 Ag1s2 + Zn2+1aq2 ∆ 2 Ag+1aq2 + Zn1s2(f) H2O1l2 ∆ H+1aq2 + OH-1aq2(g) 2 H2O1l2 ∆ 2 H+1aq2 + 2 OH-1aq2Oligomeric proteins are common and commonly occur when binding a ligand. Consider a reaction where 2 molecules of A combine with B to form an A2B complex. No detectable AB complexes exist. Using your knowledge of equilibrium binding, show that the fraction of B bound (ie fraction of A2B from the total B) fits the following expression: fraction B bound = [?] 2 ?? + [?] 2Determine values of Kc from the values of Kp given a. 2NO(g) + O2(g) ⇌ 2NO2(g) Kp = 1.48 x 104 at 184K b. Sb2S3(s) + 3H2 ⇌ 2Sb(s) + 3H2S(g) Kp = 0.429 at 713K
- Some sulfuric acid is spilled on a lab bench. You can neutralizethe acid by sprinkling sodium bicarbonate on it and thenmopping up the resulting solution. The sodium bicarbonatereacts with sulfuric acid according to:2 NaHCO31s2 + H2SO41aq2¡Na2SO41aq2 +2 H2O1l2 + 2 CO21g2Sodium bicarbonate is added until the fizzing due to the formationof CO21g2 stops. If 27 mL of 6.0 M H2SO4 was spilled,what is the minimum mass of NaHCO3 that must be addedto the spill to neutralize the acid?From the data given below calculate the value of ΔS° for the reaction. T = 298.15 K. A(g) + 1B(g) ---> 1C(s) + D(l)ΔfH°/(kJ mol-1) -393.23 -44.71 -332.49 -284.45ΔfG°/(kJ mol-1) -392.07 -15.56 -197.3 -231.43Write the expression for Kc for the following reactions. Ineach case indicate whether the reaction is homogeneousor heterogeneous.(a) 3 NO(g) ⇌ N2O(g) + NO2(g)(b) CH4(g) + 2 H2S(g) ⇌ CS2(g) + 4 H2(g)(c) Ni(CO)4(g) ⇌ Ni(s)+ 4 CO(g)(d) HF(aq) ⇌ H+(aq) + F-(aq)(e) 2 Ag(s) + Zn2+(aq) ⇌ 2 Ag+(aq) + Zn(s)(f) H2O(l) ⇌ H+(aq) + OH-(aq)(g) 2 H2O(l) ⇌ 2 H+(aq) + 2 OH-(aq)
- The following equilibria were attained at 823 K:CoO(s) + H2(g) ⇌ Co(s) + H2O(g) Kc = 67CoO(s) + CO(g) ⇌ Co(s) + CO2(g) Kc = 490Based on these equilibria, calculate the equilibrium constantfor H2(g) + CO2(g) ⇌ CO(g) + H2O(g) at 823 K.The vapor-liquid reactive equilibria for ethyl lactate synthesis follows the expressionlog K = 7.893 – (2.4312 x 103)K/Tbetween 83.12 °C and 101.54 °C. What are the ΔH°rxn, ΔG°rxn, and ΔS°rxn at 85 °C?Ind. Eng. Chem. Res. 2008, 47, 5, 1453–1463Physical Chemistry: Show complete solution. For the reaction 2NaHSO4(s) ⇌ Na2S2O7(s) + H2O(g) ΔH° = 19,800 cal/moland ΔG°= 9000 cal/mol at 25°C. Assuming ΔH° to be constant, calculate thedissociation pressure of NaHSO4 at 700 K.
- The dissociation of HI at 723 K has an equilibrium constant of 2.0×10–2 (unitless). a) Calculate ΔrxnG for this process.b) Write the chemical reaction and associated equilibrium expression for this process.The value of ΔS° for the catalytic hydrogenation of acetylene to ethane, C2H2 (g) + 2H2 (g) → C2H6 (g) is __________ J/K∙ mol. A) -76.0 B) +440.9 C) -232.5 D) +232.5 E) +28.7calculate Kc for the reaction PCl5 = PCl3 + Cl2 at 500K, if at equilibrium moment 54% of PCl5 is dissolved, and the initial concentration of PCl5 was 1M

