6. In class we went through a semi-empirical derivation of the i(t)-Eappi Curve expected in a voltammetry experiment involving a reversible redox reaction, 0.0592, -log- 0.0592, Flog mo. m (t) – i(t)) i(t) Eapol E mR Eq. 2-1 Show how to rearrange Eq. 2-1 to yield an expression to calculate the current i(t) for a particular Egppl value, i(1) = f(E appl ) -
6. In class we went through a semi-empirical derivation of the i(t)-Eappi Curve expected in a voltammetry experiment involving a reversible redox reaction, 0.0592, -log- 0.0592, Flog mo. m (t) – i(t)) i(t) Eapol E mR Eq. 2-1 Show how to rearrange Eq. 2-1 to yield an expression to calculate the current i(t) for a particular Egppl value, i(1) = f(E appl ) -
Chapter22: Bulk Electrolysis: Electrogravimetry And Coulometry
Section: Chapter Questions
Problem 22.30QAP
Related questions
Question
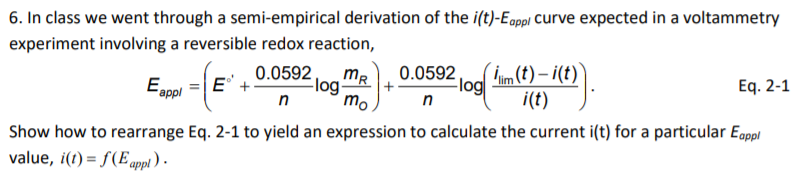
Transcribed Image Text:6. In class we went through a semi-empirical derivation of the i(t)-Eappl Curve expected in a voltammetry
experiment involving a reversible redox reaction,
0.0592,
mR
m(t) – i(t)
0.0592
Eappl =|E+
Flog-
mo
-log
n
Eq. 2-1
n
i(t)
Show how to rearrange Eq. 2-1 to yield an expression to calculate the current i(t) for a particular Egppl
value, i(t) = f(E appl ) -
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning