6. The vapour pressure of water and propanol are 71.9 and 74.0 torr respectively. The vapor pressures of several solutions of water-propanol (CH3CH2CH2OH) were determined at various compositions, with the following data collected at 45°C: Vapour Pressure (torr) XH20 74.0 0.15 77.3 a) Are solutions of water and propanol ideal, or a positive or negative deviation from Raoult's Law? Explain. b) Predict the sign of AHsoln for water-propanol solutions. 0.37 80.2 0.54 81.6 c) Are the interactive forces between propanol and water molecules weaker than, stronger than, or equal to the interactive forces between the pure substances? Explain. 0.69 80.6 0.83 78.2 1.00 71.9 d) Which of the solutions in the data would have the lowest normal boiling point?
6. The vapour pressure of water and propanol are 71.9 and 74.0 torr respectively. The vapor pressures of several solutions of water-propanol (CH3CH2CH2OH) were determined at various compositions, with the following data collected at 45°C: Vapour Pressure (torr) XH20 74.0 0.15 77.3 a) Are solutions of water and propanol ideal, or a positive or negative deviation from Raoult's Law? Explain. b) Predict the sign of AHsoln for water-propanol solutions. 0.37 80.2 0.54 81.6 c) Are the interactive forces between propanol and water molecules weaker than, stronger than, or equal to the interactive forces between the pure substances? Explain. 0.69 80.6 0.83 78.2 1.00 71.9 d) Which of the solutions in the data would have the lowest normal boiling point?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter12: Solutions
Section: Chapter Questions
Problem 12.84QE
Related questions
Question
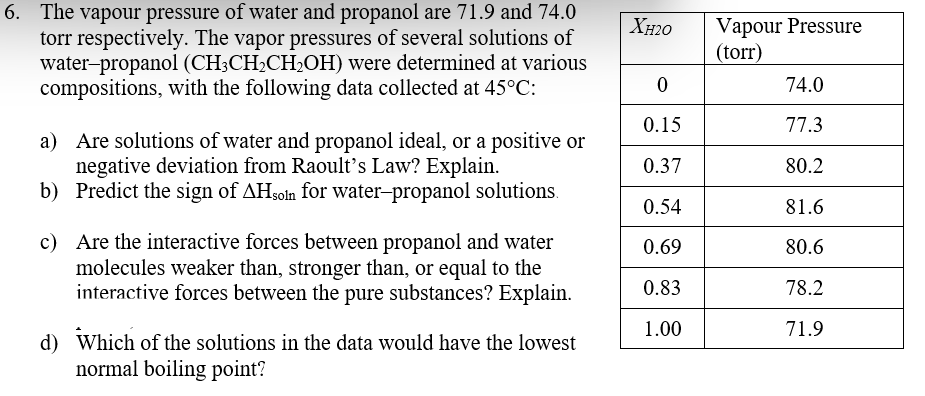
Transcribed Image Text:6. The vapour pressure of water and propanol are 71.9 and 74.0
torr respectively. The vapor pressures of several solutions of
water-propanol (CH3CH2CH2OH) were determined at various
compositions, with the following data collected at 45°C:
Vapour Pressure
(torr)
XH20
74.0
0.15
77.3
a) Are solutions of water and propanol ideal, or a positive or
negative deviation from Raoult's Law? Explain.
b) Predict the sign of AHsoln for water-propanol solutions.
0.37
80.2
0.54
81.6
c) Are the interactive forces between propanol and water
molecules weaker than, stronger than, or equal to the
interactive forces between the pure substances? Explain.
0.69
80.6
0.83
78.2
1.00
71.9
d) Which of the solutions in the data would have the lowest
normal boiling point?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning