6. Using phase diagram for the mixtures of benzene and ethylbenzene shown below, estimate: Benzene / Ethybenzene at 1.00 atm By NRTL A) Boiling point of pure ethylbenzene 140- 135- 130- B) Boiling point (Tpp) of solution with molar fraction of benzene 0.3. 125 120- 115 110- C) Composition of vapor phase (molar fractions of both liquids) above the solution with molar fraction of benzene 0.3 at Töp. 105- 100 90 85 80- 75- a1 02 03 04 05 06 0.7 0.8 09 Benzene Mole Fracfioni
6. Using phase diagram for the mixtures of benzene and ethylbenzene shown below, estimate: Benzene / Ethybenzene at 1.00 atm By NRTL A) Boiling point of pure ethylbenzene 140- 135- 130- B) Boiling point (Tpp) of solution with molar fraction of benzene 0.3. 125 120- 115 110- C) Composition of vapor phase (molar fractions of both liquids) above the solution with molar fraction of benzene 0.3 at Töp. 105- 100 90 85 80- 75- a1 02 03 04 05 06 0.7 0.8 09 Benzene Mole Fracfioni
Chapter84: Fractional Distillation, Azeotropes
Section: Chapter Questions
Problem 5P
Related questions
Question
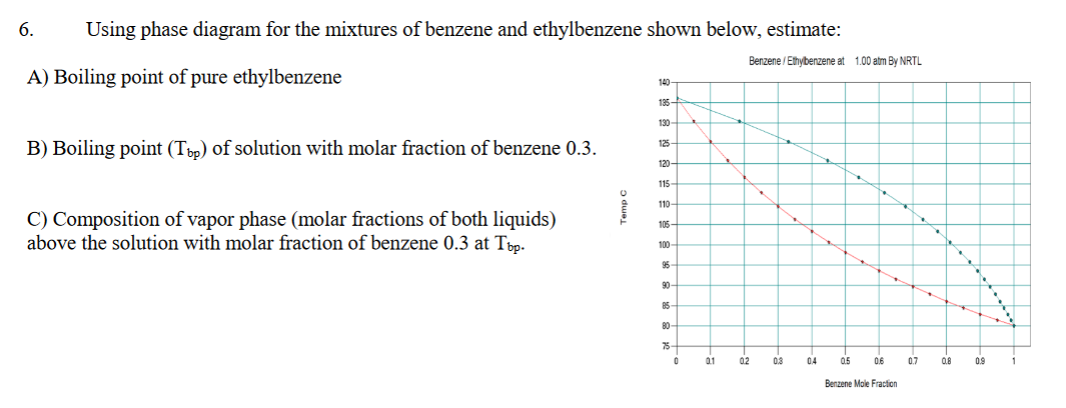
Transcribed Image Text:6.
Using phase diagram for the mixtures of benzene and ethylbenzene shown below, estimate:
Benzene / Ethybenzene at 1.00 atm By NRTL
A) Boiling point of pure ethylbenzene
140-
135-
130-
B) Boiling point (Tpp) of solution with molar fraction of benzene 0.3.
125
120-
115
110-
C) Composition of vapor phase (molar fractions of both liquids)
above the solution with molar fraction of benzene 0.3 at Töp.
105-
100
90
85
80-
75-
a1
02
03
04
05
06
0.7
0.8
09
Benzene Mole Fracfioni
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
