a. What is the chemical formula of the solid represented by the vertical phase boundary passing through point g? (Hint: use mole fractions of A and B)
a. What is the chemical formula of the solid represented by the vertical phase boundary passing through point g? (Hint: use mole fractions of A and B)
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.57E
Related questions
Question
a. What is the chemical formula of the solid represented by the vertical phase boundary passing through point g? (Hint: use mole fractions of A and B)
b. Suppose we have a solid mixture represented by point k. How much of the solid in letter a is in equilibrium per kg of pure B?
Given the Txy diagram below of a two-component (A and B) solid-liquid system.
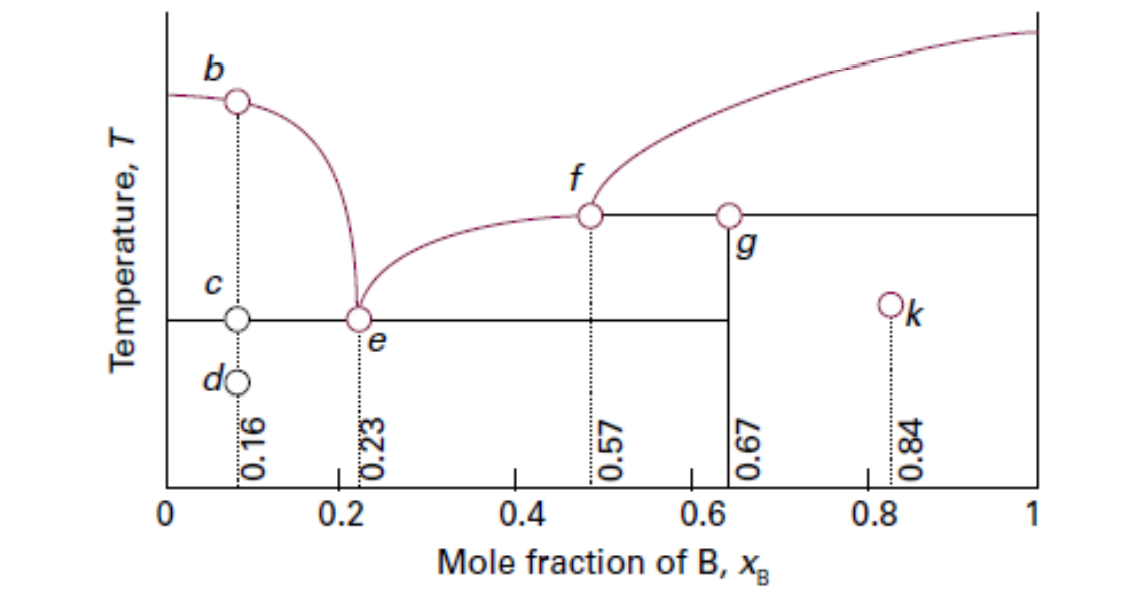
Transcribed Image Text:g
Ok
e
0.2
0.4
0.6
0.8
1
Mole fraction of B, xg
Temperature, T
0.16
0.23
0.57
0.67
0.84
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,