3. Draw (illustrate) a hypothetical phase diagram incorporating the following salient features: A. presence of eutectic point B. presence of peritectic point C. compound formation Label the axis and the points. From your diagram, estimate the composition of your new compound 4. In the making of ice cream at -10°C, the phase diagram ON THE RIGHT is essential in knowing the optimized condition for its production. Given the phase diagram of sucrose aqueous solution, at what condition (0%, 20% or 54%) will you have the best ice cream mix after freezing. Explain the basis of your choice. 160 120 0- adhs saturated solution 40- kend liquid lass 20 40 60 100 Sucrose Cantent (% by weight) Temperature C)
3. Draw (illustrate) a hypothetical phase diagram incorporating the following salient features: A. presence of eutectic point B. presence of peritectic point C. compound formation Label the axis and the points. From your diagram, estimate the composition of your new compound 4. In the making of ice cream at -10°C, the phase diagram ON THE RIGHT is essential in knowing the optimized condition for its production. Given the phase diagram of sucrose aqueous solution, at what condition (0%, 20% or 54%) will you have the best ice cream mix after freezing. Explain the basis of your choice. 160 120 0- adhs saturated solution 40- kend liquid lass 20 40 60 100 Sucrose Cantent (% by weight) Temperature C)
Chapter84: Fractional Distillation, Azeotropes
Section: Chapter Questions
Problem 5P
Related questions
Question
1o
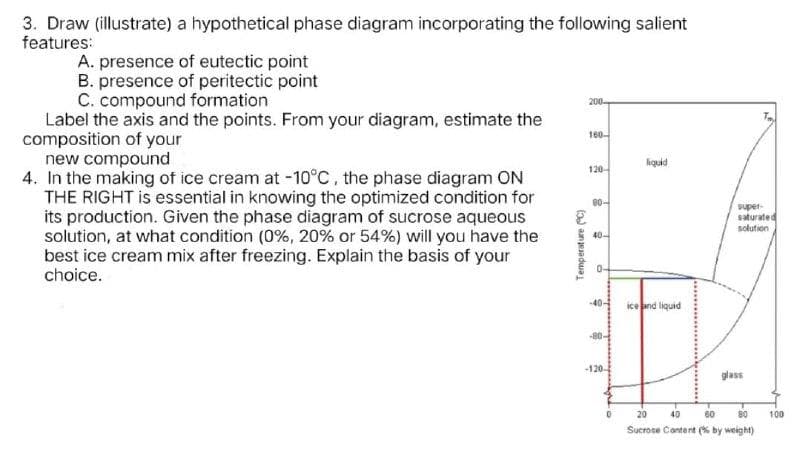
Transcribed Image Text:3. Draw (illustrate) a hypothetical phase diagram incorporating the following salient
features:
A. presence of eutectic point
B. presence of peritectic point
C. compound formation
Label the axis and the points. From your diagram, estimate the
composition of your
new compound
4. In the making of ice cream at -10°C, the phase diagram ON
THE RIGHT is essential in knowing the optimized condition for
its production. Given the phase diagram of sucrose aqueous
solution, at what condition (0%, 20% or 54%) will you have the
best ice cream mix after freezing. Explain the basis of your
choice.
200-
160-
kquid
120-
80-
supet-
saturated
solution
S 40-
0-
-40-
ice and liquid
-80-
-120-
glass
20
40
60
80
100
Sucrose Content (% by weight)
Temperature C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,