6.312 g of p-aminophenol (MW = 109.13 g/mol) is reacted with 6.600 g of acetic anhydride (MW = 102.09 g/mol) to form acetaminophen (151.16 g/mol) and acetic acid (60.052 g/mol) as shown in the reaction below. What is the theoretical yield of acetaminophen? CH3 NH2 HC NH HC + CH H3C но CH3 CH но CH3 но p-aminophenol 11.95 g 9.772 g acetic anhydride acetaminophen acetic acid O 9.258 g 8.743 g 13.14 g
6.312 g of p-aminophenol (MW = 109.13 g/mol) is reacted with 6.600 g of acetic anhydride (MW = 102.09 g/mol) to form acetaminophen (151.16 g/mol) and acetic acid (60.052 g/mol) as shown in the reaction below. What is the theoretical yield of acetaminophen? CH3 NH2 HC NH HC + CH H3C но CH3 CH но CH3 но p-aminophenol 11.95 g 9.772 g acetic anhydride acetaminophen acetic acid O 9.258 g 8.743 g 13.14 g
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter22: Organic And Biological Molecules
Section: Chapter Questions
Problem 157CP
Related questions
Question
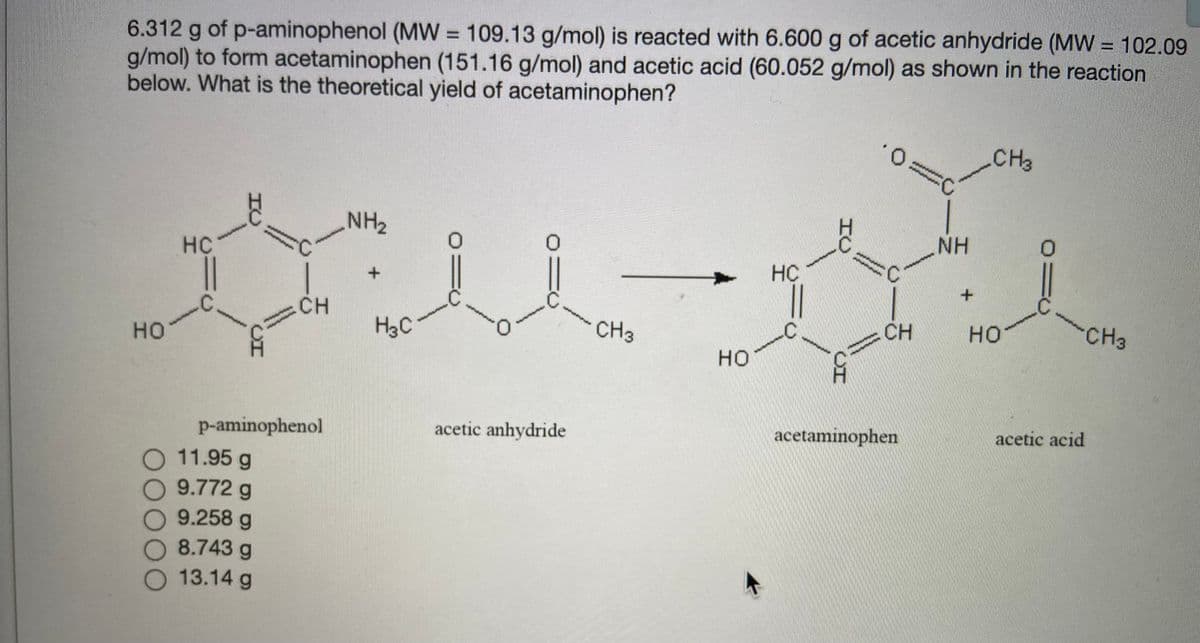
Transcribed Image Text:6.312 g of p-aminophenol (MW = 109.13 g/mol) is reacted with 6.600 g of acetic anhydride (MW = 102.09
g/mol) to form acetaminophen (151.16 g/mol) and acetic acid (60.052 g/mol) as shown in the reaction
below. What is the theoretical yield of acetaminophen?
%3D
O.
CH3
%3D
H.
NH2
NH
HC
HC
CH
CH
но
CH3
CH3
H3C
но
но
acetic acid
acetaminophen
acetic anhydride
p-aminophenol
O 11.95 g
9.772g
9.258 g
O 8.743 g
O 13.14 g
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.