6.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 60. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 9.54 g water 3.90 g olo Use this information to find the molecular formula of X.
6.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of 60. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured: product mass carbon dioxide 9.54 g water 3.90 g olo Use this information to find the molecular formula of X.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 24PS: Methanol, CH3OH, can be prepared from carbon monoxide and hydrogen. CO(g) + 2 H2(g) CH3OH(l) What...
Related questions
Question
6.5 g of a certain Compound
X
, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular molar mass of
60./gmol
, is burned completely in excess oxygen, and the mass of the products carefully measured:
| product | mass |
| carbon dioxide |
9.54g
|
| water |
3.90g
|
Use this information to find the molecular formula of
X
.
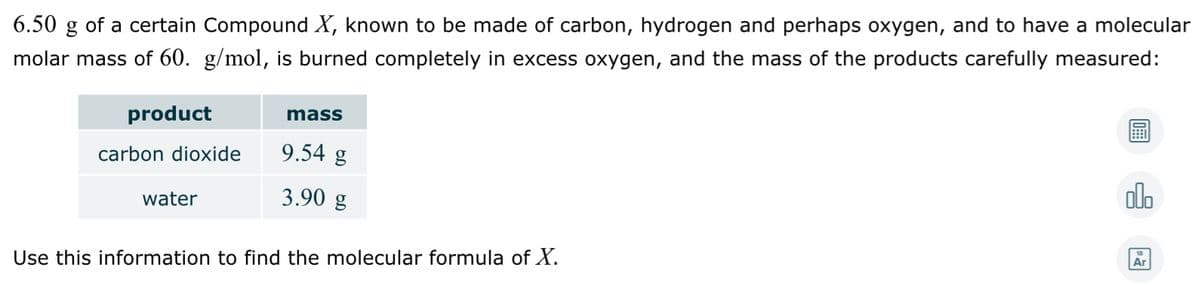
Transcribed Image Text:6.50 g of a certain Compound X, known to be made of carbon, hydrogen and perhaps oxygen, and to have a molecular
molar mass of 60. g/mol, is burned completely in excess oxygen, and the mass of the products carefully measured:
product
mass
carbon dioxide
9.54 g
3.90 g
alo
water
Use this information to find the molecular formula of X.
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning